Difference Between Gold and Rose Gold
What is Gold?
Gold is a metal known for its whitish yellow appearance. It is also a mineral in its elemental form making it a native mineral. As a mineral, it has a metallic luster, yellowish streak, and hackly fracture. It has been used by humans for thousands if not tens of thousands of years.
Chemistry behind Gold
Gold is one of the heavy elements. It has an atomic number of 79. Gold also only has one naturally occurring stable isotope, 197Au. Other isotopes of gold can be produced, but all other isotopes of gold are radioactive. The most stable isotope of gold other than 197Au is 195Au which has a half-life of about 186 days.
Gold has many properties that make it unique. It is malleable enough that it can be made into variety of shapes. It can be made into a broad sheet and into a wire, for example. It is also a good conductor of electricity. Additionally, it is generally non-reactive and will not tarnish.
Gold is too heavy to have formed in stars during the normal process of nucleosynthesis. The heaviest element that can be produced in the core of a star is iron which has an atomic number of only 26. Gold most likely formed through supernova nucleosynthesis. There is also a possibility that gold is formed in the event of collisions between neutron stars. Most gold which was incorporated into Earth when it formed, most likely sank to the core. As a result, most gold in the crust is probably from asteroid impacts. Abundant gold deposits found near known impact craters supports this though they don’t necessarily confirm it.
On Earth’s surface, gold is most commonly found in hydrothermal quartz veins, certain volcanic deposits, and placer deposits. Gold can be washed out into alluvial fans where it will form into nuggets. The discovery of gold nuggets is famous for beginning many gold rushes.
Reasons why Gold is used by Humans
Gold was probably one of the first metals to be used by humans since it can be found easily in its natural form and used without being extracted or smelted. There is also evidence of it having been used around 40,000 B.C. in Paleolithic Europe. The first civilizations to use gold to craft jewelry, as opposed to using it in its natural state, may have been ancient Mesopotamia and Egypt during the 3rd millennium B.C.
Gold was first used to make currency around 700 B.C. in Lydia. These early coins were made of a mixture of gold and electrum. Because of the natural rarity of gold and its unique properties, it has always been considered an important metal that has been associated with wealth, power, the elites, beauty, and luxury.
Outside of its use as currency, gold is also used in circuits because it is an effective conductor. Gold is also famous for being an element which dissolves in almost nothing. The only solution known to completely dissolve gold is aqua regia. Gold is also used in electrochemical batteries.
What is Rose Gold?
Rose gold is an alloy of gold and copper. When gold is alloyed with copper it gains a reddish tinge. The more copper that is in the alloy, the redder the gold-copper alloy will become. An alloy containing very little copper is called pink gold and one containing excessive amounts of copper is red gold. Rose gold contains moderate amounts of copper compared to pink gold and red gold.
When the copper content in the gold-copper alloy is less than 12%, the alloy is still quite malleable. The rigidness of the alloy increases with the copper content. Gold-copper alloys have been used to make coins for centuries since gold-copper alloys are easier to come by than pure gold, though some coins such as florins and byzants in the Middle Ages were almost pure gold. Today, rose gold and other copper-gold alloys are popular in jewelry. In ancient times, it was difficult to obtain pure gold. As a result, gold would often contain many impurities. Gold tinted with copper was common in the ancient Near East. Because of this, gold used in ancient times often had a reddish tinge.
Gold-copper alloys were also common in parts of Africa. Africa is rich in iron and gold but poor in copper, as a result, copper was considered more valuable in many cultures in Sub-Saharan Africa. As a result, gold appears to have been often combined with copper to create gold-copper alloys. Pure gold only came into widespread use because of the influence of European and Arabian traders.
Similarities between gold and rose gold
Gold and rose gold are both malleable and rare. They also contain varying degrees of the element gold. Similarly, they have both been used in currency and jewelry.
Differences between gold and rose gold
Although there are similarities between the two metals, there are also differences which include the following.
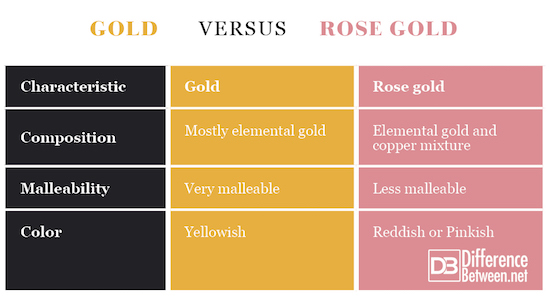
- Rose gold is an alloy of two metals whereas pure gold is just one metal though gold can have varying amounts of impurities.
- Pure gold is more malleable than copper-contaminated rose gold
- Gold has a yellowish color whereas rose gold is reddish or pinkish
Gold vs Rose Gold: Comparison Chart
Summary of Gold Vs. Rose Gold
Gold is a typically yellow heavy metal. It is known for being rare, malleable, and nonreactive. Because of its nonreactivity, it is sometimes used in electrochemical batteries. Gold is one of the oldest metals to be used by humans. Gold only has one stable isotope in nature, 197Au. All other isotopes are radioactive and have half-lives too short to be common. The most stable radioactive isotope of gold is 195Au with a half-life of 186 days. Rose gold is an alloy of gold and copper. The copper gives it a reddish tinge and the malleability of rose gold decreases with increasing copper content. Like pure gold, rose gold has been used in currency and is popular in jewelry.
- Difference Between Environmental Performance Index and Development - November 24, 2023
- Difference Between Environmental Intervention and Development - November 8, 2023
- Difference Between Eco Efficiency and Eco Effectiveness - September 18, 2023
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://www.maxpixel.net/Gold-Rose-Gold-Commitment-Wedding-Ring-Rings-2807717
[1]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/6/60/Gold_Ingots_on_white_background.jpg/640px-Gold_Ingots_on_white_background.jpg
[2]Rose, Thomas Kirke. The metallurgy of gold. Vol. 2. C. Griffin, limited, 1898.
[3]Herbert, Eugenia W. Red gold of Africa: Copper in precolonial history and culture. Univ of Wisconsin Press, 1984.
[4]Gopher, Avi, et al. "Earliest gold artifacts in the Levant." Current Anthropology 31.4 (1990): 436-443.
[5]Abbott, Benjamin P., et al. "GW170817: observation of gravitational waves from a binary neutron star inspiral." Physical Review Letters 119.16 (2017): 161101.
[6]Gimeno, M. Concepción. "The chemistry of gold." Modern Supramolecular Gold Chemistry: Gole-Metal Interactions and Applications (2008): 1-63.