Difference Between Colloid and Suspension
Dispersion systems consist of two or more chemical compounds or simple substances, called system components, distributed among each other. They form:
- Dispersed phase – the dispersed substance;
- Continuous medium – the substance in which the dispersed phase is distributed.
Depending on the size of the particles of the disperse phase there are:
- Heterogenous (rough) dispersion systems – the particles are bigger than 100 nm:
- Suspension – a liquid and solid component;
- Emulsion – two liquid components;
- Aerosol – the dispersion medium is a gas.
- Colloids – the particles’ size is between 1 and 100 nm;
- Real solutions – the particle size is less than 1 nm.
What is Colloid?
Water solutions of many substances (sugar, etc.), easily pass through plant or animal semipermeable barriers, while others such as gelatin do not pass through them. The first substances are called crystalloids, and the second are called colloids.
Depending on how the particles of the dispersed phase refer to the medium, the colloid systems are:
- Lyophilic – adsorb a large number of molecules from the dispersion medium (gelatin, soaps, Fe(OH)3, Al(OH)3);
- Lyophobic – do not bind or bind with a small number of molecules from the dispersion medium (salts of certain metals, poorly soluble metal sulphides, etc.).
Depending on the colloid particle structure the colloid systems are subdivided into:
- Associated (micellar) – the particles are groups of atoms, ions or molecules (e.g. sodium chloride in benzene);
- Molecular – the particles are molecules of a compound having a high molecular mass (e.g. starch).
Depending on the nature of the medium, the colloids are:
- Hydrosols – the solvent is water;
- Benzenosols – the solvent is benzene;
- Etherosols – the solvent is ether etc.
The optical properties of the colloids are manifested as coloring, opalescence, and Tindal effect. They are due to differences in the absorption and dispersion of light from the colloidal particles.
Colloidal particles are larger and heavier than the ions and most of the molecules, so their diffusion and osmotic pressure are low.
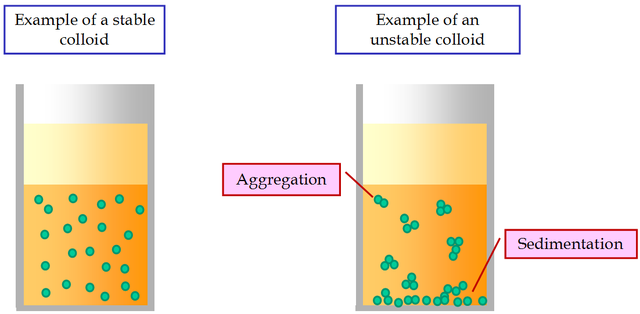
A characteristic kinetic property of colloids is the Brownian movement. The colloid systems are less stable than that of the ordinary solutions. Under a constant electric current, all the colloid particles move to the corresponding oppositely charged electrode. This phenomenon is called electrophoresis.
Sols of molecular colloids are obtained analogously to the actual solutions. Upon contact of the disperse phase dissolves spontaneously in the dispersed medium. The sols of associated colloids are obtained by various dispersion and condensation methods.
- Dispersion methods – dispersion of the material to the size of the colloidal particles in the presence of a dispersion medium;
- Condensation methods – condensing (grouping) individual molecules, atoms or ions into particles of colloid size.
What is Suspension?
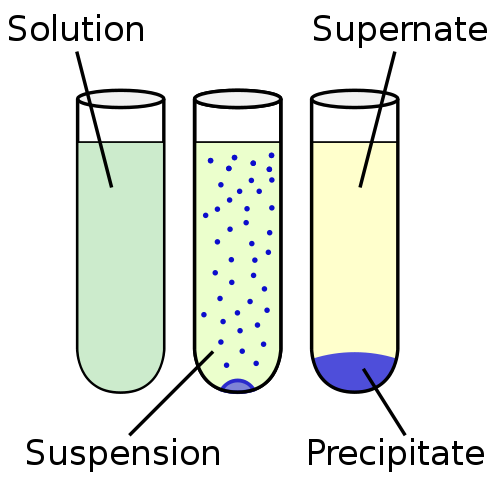
Suspension is a heterogeneous liquid, containing insoluble solid particles that are large enough to settle but for some time are present throughout the volume of the liquid matrix. The particles are bigger than 100 nm.
The classification of the suspensions is based on the dispersed phase and the dispersion medium.
The suspension is closer to the insolubility in the solubility continuum. In the other end of the solubility continuum is the solution, where the particles are completely mixed and no solid phase is observed. The solubility continuum is generally arranged in the order: insolubility, sedimentation, suspension, colloid and solution.
The solid phase of the suspension is dispersed in the liquid phase by a mechanical stirring process by means of an inert or weakly active agent used as a suspending agent. Unlike colloids, the suspensions settle down over time. An example of a rapidly precipitating suspension is sand and water.
A characteristic property of the suspensions is their optical inhomogeneity, which is expressed by turbidity. Turbidity is an integral external sign of the suspension and is determined by the presence of insoluble particles that are impermeable to light. The degree of turbidity of suspensions is different. It is determined by the concentration of the suspended phase and the degree of its dispersion (particle size).
One of the most important features of the suspensions is their sedimentation instability. It is expressed in the inevitable settling of suspended particles under the influence of gravity. Particles can settle by themselves, without sticking together. In this case there is an aggregative stability of the suspension.
If the settling particles stick together under the influence of molecular forces of cohesion and form aggregates, then there is an aggregative instability of suspensions. Thus, sedimentationally unstable suspensions can be aggregatively stable or unstable.
Sometimes in coagulating suspensions, large flakes are formed that are poorly wetted by the dispersion medium and float to the surface. This phenomenon is called flocculation.
Sedimentation instability of suspensions in practice leads to a gradual disruption of the uniform composition before the complete deposition of the insoluble phase.
There are also suspensions, having the ability to remain in a suspended state for a long time. They are called stable suspensions.
The suspensions are obtained by various dispersion and condensation methods.
Difference Between Colloid and Suspension
-
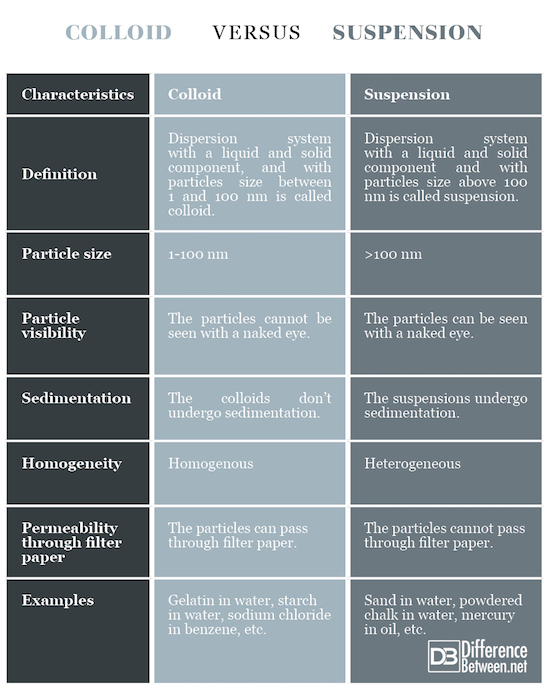
Definition
Colloid: Dispersion system with a liquid and solid component, with particles size between 1 and 100 nm is called colloid.
Suspension: Dispersion system with a liquid and solid component, with particles size above 100 nm is called suspension.
-
Particle size
Colloid: The particle size is 1-100 nm.
Suspension: The particle size is above 100 nm.
-
Particle visibility
Colloid: The particles in the colloid cannot be seen with a naked eye.
Suspension: The particles in the suspension can be seen with a naked eye.
-
Sedimentation
Colloid: The colloids don’t undergo sedimentation.
Suspension: The suspensions undergo sedimentation.
-
Homogeneity
Colloid: The colloids are relatively homogenous.
Suspension: The suspensions are heterogeneous.
-
Permeability through filter paper
Colloid: The colloid particles can pass through filter paper.
Suspension: The suspension particles cannot pass through filter paper.
-
Examples
Colloid: Gelatin in water, starch in water, sodium chloride in benzene, etc.
Suspension: Sand in water, powdered chalk in water, mercury in oil, etc.
Colloid and Suspension Comparison Chart
Summary of Colloid and Suspension
- Dispersion systems consist of two or more chemical compounds or simple substances, called system components, distributed among each other. They form a dispersed phase and a continuous medium.
- Dispersion system with a liquid and solid component, with particles size between 1 and 100 nm is called colloid.
- Dispersion system with a liquid and solid component, with particles size above 100 nm is called suspension.
- The particles in the colloid cannot be seen with a naked eye, while the particles in the suspension can be seen with a naked eye.
- The colloids don’t undergo sedimentation, while the suspensions undergo sedimentation.
- The colloids are relatively homogenous, while the suspensions are heterogeneous.
- The colloid particles can pass through filter paper, while the particles of the suspensions cannot.
- Examples of colloids are gelatin in water, starch in water, sodium chloride in benzene, etc. Examples of suspensions are sand in water, powdered chalk in water, mercury in oil, etc.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
2 Comments
Leave a Response
References :
[0]De, A. A Text Book of Inorganic Chemistry. New Delhi: New Age Publishers. 2003. Print.
[1]Housecroft, C., A. Sharpe. Inorganic Chemistry. London: Pearson Education Limited. 2012. Print.
[2]Lazarov, D. Inorganic Chemistry. Sofia: St. Kliment Ohridski. 1995. Print.
[3]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/7/78/Chemical_precipitation_diagram.svg/500px-Chemical_precipitation_diagram.svg.png
[4]Image credit: https://commons.wikimedia.org/wiki/File:ColloidalStability.png

I like your views on this topic
Nice lesson ,I really appreciate alot .I have able to gain understanding for some of the questions.