Difference Between Electrophile and Nucleophile
Electrophile and Nucleophile are the two important concepts in organic chemistry that help describe the chemical reactions between electron acceptors and donors. These two terms were introduced in 1933 by Christopher Kelk Ingold and they served as replacements for cationoid and anionoid terms which were introduced in 1925 by A.J. Lapworth.
Since then, extensive studies were undertaken to understand the different between electrophile and nucleophile. This article demystifies the difference between these two concepts. In a nutshell, a nucleophile is an electron donor whereas an electrophile is an electron acceptor.
What is an Electrophile?
To breakdown the term, the word “electro” is from electrons and the Latin word “phile” refers to “loving”. In simple terms, it means electrons-loving. It is a reagent that is characterized with a low density of electrons in its valance shell, and, therefore, reacts with a high-density molecule, ion or atom to form a covalent bond. Hydrogen ion in acids and methyl-carbocation are examples of electrophilic substances. They are electron deficient.
An electrophile is easily detected by a positive charge or neutral charge with empty orbitals (not satisfying the octet rule). Electrons move from an area of high density to the one with low density, and unlike charges attract each other. This theory explains the attraction of electrons by the electron-deficient electrophile atoms, molecules or ions. By definition, an electrophile is interchangeably called a Lewis acid as it accepts electrons in line with the definition of the acid.
The reaction and compounds below show the examples of electrophiles:
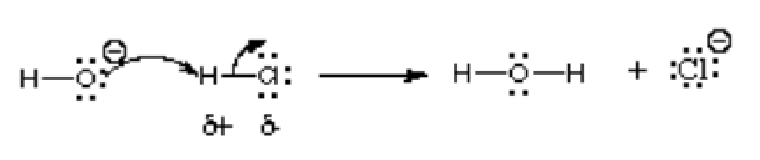
In this reaction, hydroxide ion is reacting with hydrogen chloride; thus an acid is reacting with a base. As indicated by the arrow, the more electronegative oxygen atom donates electrons to the electron-deficient hydrogen atom. It shares a lone pair to the hydrogen atom which bears a positive charge in the compound hydrogen chloride because is more electronegative than hydrogen. This reaction is a fundamental of many organic chemistry reactions, particularly Lewis acid and Lewis base reactions. Other examples are depicted in the following picture:
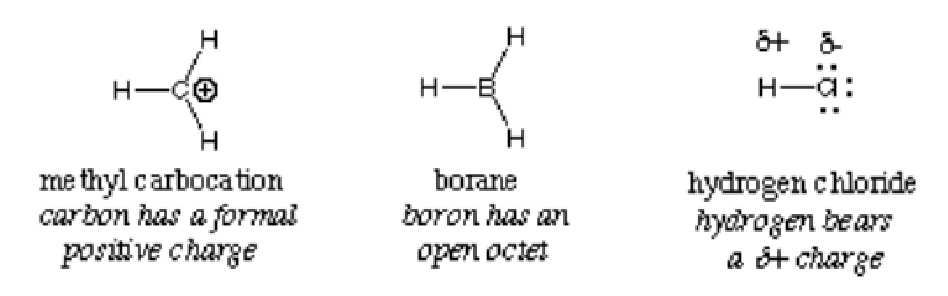
In general, an electrophile is identified by a partial positive charge as in hydrogen chloride, a formal positive charge as in methyl carbocation or vacant orbitals. Polarized neutral molecules such as acyl halides, carbonyl compounds, and alkyl halides are typical examples of electrophiles.
Important: Hydronium ion, although it bears a positive charge, does not qualify to be classified as an electrophile due to the full vacant orbitals in its outer shell. It yields hydrogen ion and water. The same applies to the ammonium ion; it does not have vacant orbitals that can attract electrons. As a result, it is not an electrophile.
What is a nucleophile?
The term is broken down into the word “nucleo” which refers to the nucleus and the Latin word “phile” which means loving. It simply means nucleus loving. Nucleophiles are rich in electrons and, as thus, donate electron pairs to electrophiles to form covalent bonds in chemical reactions. These substances are best noticed with lone pairs, pi bonds and negative charges. Ammonia, iodide and hydroxide ions are examples of nucleophile substances.
By definition, a nucleophile is interchangeably called the Lewis base because they all donate electrons and accept protons. The picture below depicts the examples of nucleophiles:
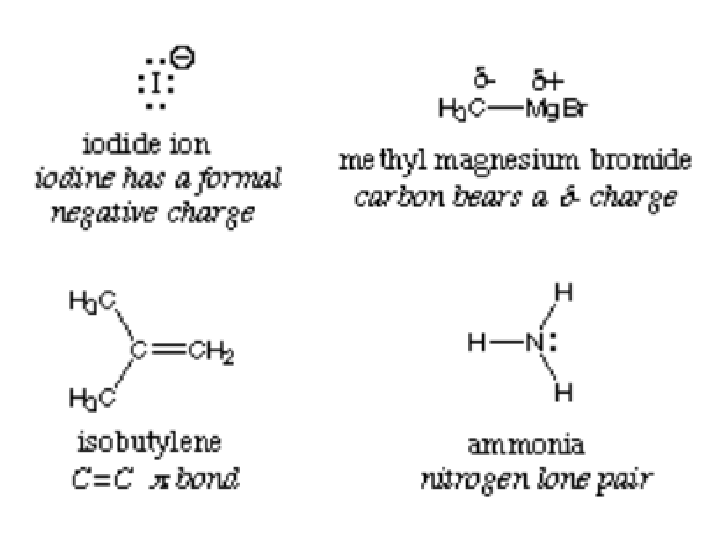
The nucleophilic center in a compound is detected with the most electronegative atom. Consider ammonia NH3; the nitrogen is more electronegative and thus draws electrons to the center. The compound has high electron density and, when reacting with an electrophile, say water, it donates electrons. H2O can act both as the electrophile or the nucleophile depending on the compound or molecule it reacts with.
Consider the picture below:
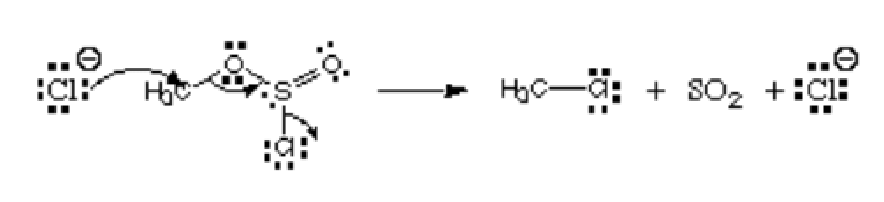
From the picture, the first atom, chloride ion is donating its lone pair to carbon to form a covalent bond. It has the negative charge and donates electrons, and so it is regarded as the nucleophile. That chlorine atom that is leaving the chlorosulfite ester is named the leaving group. It is not an electrophile or a nucleophile.
Key difference between electrophile and nucleophile
Definition of electrophile and nucleophile
An electrophile is a Lewis acid that accepts electrons from an electron-rich atom, ion or molecule. By accepting electrons, it forms a covalent bond. This reagent often identified by partial positive charge, formal positive charge or a neutral atom, ion or molecule that does not satisfy the octet rule. A nucleophile, on the other hand, is an atom, ion or molecule that has a high density of electrons. It donates a lone pair to the electrophile to form a covalent bond. It is identified by positive charges and free electrons in its orbital.
Chemical Reactions of electrophile and nucleophile
A nucleophile is involved in nucleophilic substitution and addition whereas an electrophile is involved in an electrophilic substitution and addition.
Charge Identity in electrophile and nucleophile
An electrophile can be neutrally or positively charged whereas the nucleophile can be neutrally or negatively charged. An electrophile accepts electrons hence it is referred to as the Lewis acid whereas a nucleophile donates electrons hence it is referred to as the Lewis base.
Electrophile Verses Nucleophile : Comparison Chart
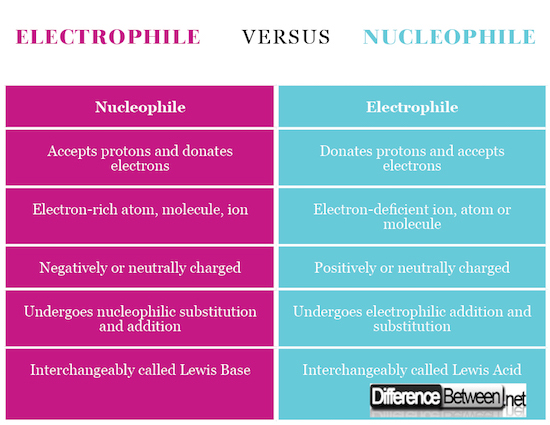
Summary of Electrophile Verses Nucleophile
- An electrophile is an electron-deficient atom,, ion or molecule while the nucleophile is an electron-rich atom, molecule or ion
- An electrophile can be positively or neutrally charged while the nucleophile can be negatively or neutrally charged
- An electrophile is called the Lewis acid and the nucleophile is called the Lewis base
- An electrophile accepts electrons and donates protons while a nucleophile donates electrons and accepts protons.
- Difference Between CBD and Indica - April 22, 2019
- Difference Between Unilateral Contract and Bilateral Contract - February 8, 2019
- Difference Between Polki and Kundan - December 15, 2018
Search DifferenceBetween.net :
 Email This Post
: If you like this article or our site. Please spread the word. Share it with your friends/family.
Email This Post
: If you like this article or our site. Please spread the word. Share it with your friends/family.
3 Comments
Leave a Response
References :
[0]James (Feb 28, 2018). Nucleophiles and Electrophiles. Accessed on: https://www.masterorganicchemistry.com/2012/06/05/nucleophiles-and-electrophiles/
[1]John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. Menlo Park, CA


Nice
It was helpful
Very useful thank you