Difference Between Heat and Thermal Energy
What is energy? It may seem like a simple question, but the answer to which is not so simple at all. This is one of the many science questions that are as fascinating as they are complex. In science, energy is the ability to do work, to make things happen how they happen and to cause changes to an object. Simply put, energy is how things change and move. Energy is everything and everywhere; in fact, it’s one of the true constants of the universe and they come in lots of different forms like sound and light, which may seem different to us. Heat is also a form of energy. The warmth from the Sun or a gas stove are examples of heat energy which is also called thermal energy.
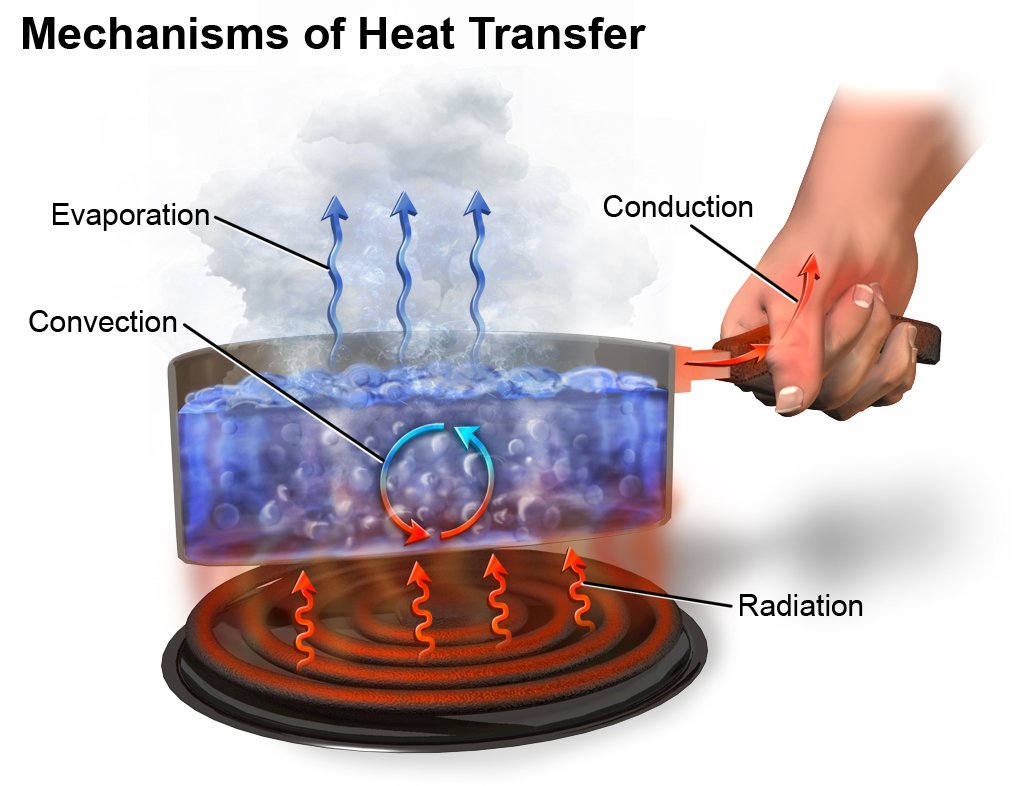
What is Heat/Heat Energy?
So, what is heat? Heat is the form of energy that flows from one object to another with different temperatures. For example, let’s say you have a material of hot body and a material of cold body. When you put them together, the energy gets transferred from the hot body into the cold body. You cannot just sense heat by simply looking at an object; you need to touch it to feel it. Like when you touch a bowl full of hot water, you can feel the heat. Heat is simply the transfer of energy from one body to another. For example, when you’re cooking something and turn on the gas, the heat gets transferred from the fire to the container, and then from the container it goes into the food and the food is cooked from that energy. Heat energy transfers from higher temperature objects to lower temperature objects. Heat is a form of energy in transit, when there is a difference in temperatures between the two objects.
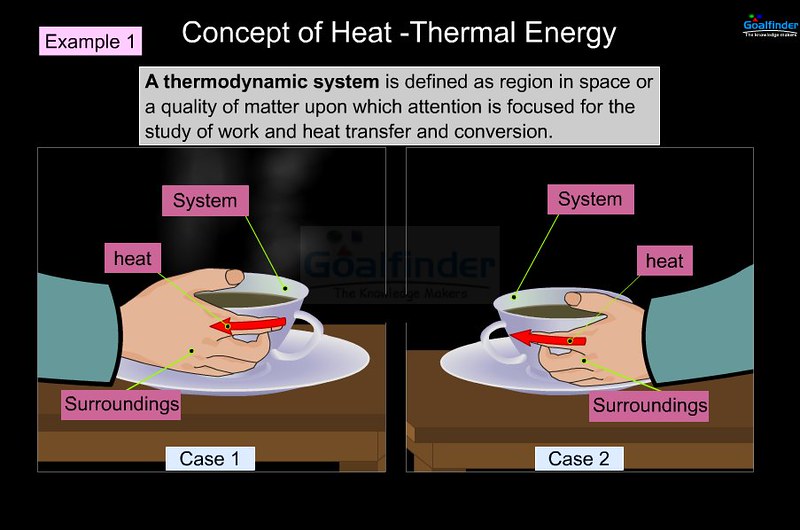
What is Thermal Energy?
Thermal energy, also refers to as heat energy, is the most basic form of energy responsible for random movements of the molecules within an object or a system. It is the energy contained within a system that makes the atoms and molecules to move faster. Consider, for example a hot object and a cold object. We all know that a hot object carries large thermal energy and a cold object carries small thermal energy. It is simply like rubbing your hands together on a freezing winter day, which converts the kinetic energy of your hands into thermal energy due to friction between your hands. Thermal energy has been used for simple tasks such as heating, boiling water and cooking food since the beginning of the human race. Thermal energy is the energy contained within a system due to the random motion of molecules that is responsible for its temperature.
Difference between Heat and Thermal Energy
Definition of Heat vs Thermal Energy
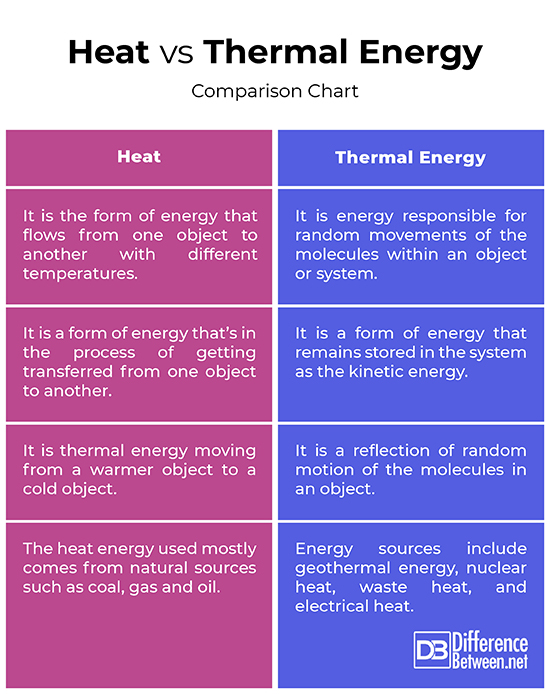
– Heat is a form of energy in transit that flow from one object to another with different temperatures. Heat is the degree of hotness or degree of coldness of an object or an environment. You cannot just sense heat by simply looking at an object; you need to touch it to feel it. Thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. Thermal energy in transit produced heat, whereas heat energy is the flow of thermal energy between two bodies at different temperatures.
Property in Heat vs Thermal Energy
– In engineering context, the term heat is often used synonymously with thermal energy. Heat is one of the oldest and most basic sources of energy known to man. Heat is not a physical object, but a form of energy that is in the process of getting transferred from one object to another with different temperatures. When you put one hot object next to a cold object, the energy gets transferred from the hot object into the cold one, until they are at equal temperatures. Thermal energy, on the other hand, is not in transit, but it’s the energy stored in the system as its kinetic energy.
Sources of Heat vs Thermal Energy
– The most important and a prominent natural source of heat energy is the Sun. Today, the heat energy used mostly comes from natural sources such as coal, gas and oil. These are solid fuels that can be stored for millions of years and created from the remains of plants and animals. When we ignite fossil fuels, heat energy is generated. Since the beginning, the primary and the main sources of the thermal energy were Sun and fire, but the technological evolution over the years have expanded the scope to new man-made energy sources such as geothermal energy, nuclear heat, waste heat, and electrical heat. Chemical energy and wind energy are other sources of thermal energy used throughout.
Heat vs. Thermal Energy: Comparison Chart
Summary
So, heat energy is a form of thermal energy trying to reach thermodynamic equilibrium while the heat gets transferred from a warmer object into a cooler one. Heat energy is a form of energy that is in transit, whereas thermal energy refers to the total energy of all the particles contained within a system and maintained at a specific temperature. Thermal energy is the random movement of atoms or molecules present in an object or a system while heat energy is the measure of the degree of hotness or coldness due to the temperature difference between two objects. So, in a nutshell, heat energy is the flow of thermal energy in a system.
- Difference Between Serif and Sans Serif - April 22, 2024
- Difference Between HTML and Text - April 19, 2024
- Difference Between FTP and SFTP - April 16, 2024
Search DifferenceBetween.net :
 Email This Post
: If you like this article or our site. Please spread the word. Share it with your friends/family.
Email This Post
: If you like this article or our site. Please spread the word. Share it with your friends/family.
1 Comment
Leave a Response
References :
[0]Shah, Yatish T. Thermal Energy: Sources, Recovery, and Applications. Florida, United States: CRC Press, 2018. Print
[1]Carlson-Berne, Emma. Hot!: Heat Energy. New York, United States: Rosen Publishing, 2013. Print
[2]Image credit: https://live.staticflickr.com/7177/6806304294_5dda097af9_c.jpg
[3]Image credit: https://commons.wikimedia.org/wiki/File:Heat_Transfer.png


Heat and cold could also transformed wet and watered.