Difference Between Molar Mass and Atomic Mass
Introduction
At the microscopic level, we noticed that the smallest components play a vital role shaping the macroscopic world we live into. In this article we will discuss two of the most important aspects in our world; molar mass and atomic mass. These two fundamental concepts may appear complex but they are indispensable in science. The atomic-level measurements precision holds the potential to significantly change scientific outcomes. Very small modifications at the atomic level can have great implications. This article aims to clarify these concepts and demonstrate their significance within the scientific domain.
What is Atomic Mass?
Definition
Atomic mass (ma or m) represents the total mass of an atom of a chemical element, measured in atomic mass units (amu). It quantifies the combined mass of the protons and neutrons of an atom. Protons and neutrons are both concentrated in the nucleus. As electrons have a negligible mass, they are not significantly considered in this measurement. The atomic mass of Hydrogen serves as a reference point for the other elements as it equals 1 amu.
Significance in the Atomic World
Atomic mass is a very important way to tell things apart in the huge and complicated world of atoms. Scientists can tell what an element is by its atomic mass. It is kind of like a mark for elements, telling them apart from each other. This mass controls how an atom works in chemical reactions by affecting its bonds, reactions, and interactions with other atoms. Atomic masses are carefully balanced to make compounds, which are very complicated chemical reactions.
Example from real life
Atomic mass is like the fingerprint for each element. Just like sorting stones according to their characteristics, atomic mass helps in differentiating elements from each other’s. For example, pharmacologists use atomic masses of elements to formulate different medications accurately. This ensures that the correct medical reactions will occur inside the body. Another example in astrophysics; the atomics mass informs the study of planets’ compositions and stellar phenomena. The concepts of atomic mass is not just a theoretical chemistry, it’s an important aspect of how we apply science to solve problems and improve our understanding of the universe.
What is Molar Mass?
Definition
In chemistry, molar mass is defined as the mass of one mole of a certain substance, expressed in grams per mole (g/mol). A mole is the unit that quantifies the amount of a substance, analogous to the concept of a dozen, and contains Avogadro’s number of particles (approximately 6.022 × 1023). The molar mass is calculated by summing the atomic masses of all the atoms in a molecule of the substance.
Significance in the Atomic World
Molar mass is like the link between microscopic chemistry and laboratory applications for elements. It is essential for chemists to create precise compounds, balance chemical reactions, and determine concentrations. Without molar mass, the quantitative aspect of chemistry would be highly challenging.
Example from real life
Molar mass in chemistry is like the currency in economics. Just as the currency allows the quantification of different goods, molar mass enables us to measure and exchange different chemicals accurately. Molar mass acts as a common language that translates the microscopic invisible particles into touchable quantities that can be measured and manipulated. This facilitates advancement in many different fields as pharmaceuticals, and material science.
Possible Similarities Between Atomic Mass and Molar Mass
Atomic Structure-Based:
- Both atomic and molar mass are based on the atomic structure of an element.
- They depend on the mass of protons, neutrons, and electrons (though the electron’s mass is negligible).
Reflect Mass:
- measuring atomic mass and molar mass use different scales.
Important for chemistry Calculations:
- Atomic and molecular masses are important for chemistry calculations.
- Both help us understand and guess what chemical processes will happen.
Molecular Weight Calculation:
- We use both to figure out the molecular weights of compounds.
- For particle molar mass, add up the masses of all the atoms.
Periodic Table Relevance:
- The periodic table helps you figure out values related to both atomic and molar mass.
- Finding the molar mass starts with finding the atomic masses in the periodic table.
Involvement in Theoretical and Practical Chemistry:
- Atomic and molecular mass are important in both theory and practice of chemistry, in both lab work and real-world situations.
Use in Isotopic Composition Analysis:
- Both analyze the isotopic makeup of elements.
- Finding the molar mass from the atomic mass involves looking at the average isotopic makeup.
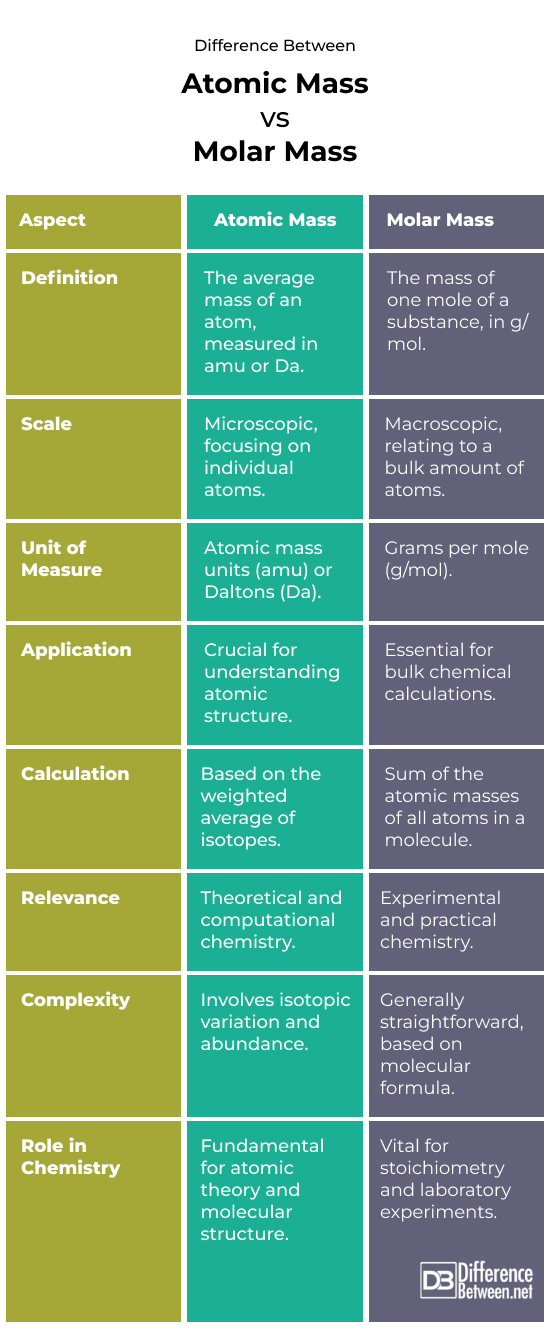
Difference Between Atomic Mass and Molar Mass
Conceptual Basis
Atomic mass comprises the average mass of all isotopes in an element.
Describes atom-by-atom constitution.
Molar Mass: The mass of one mole (Avogadro’s number of particles) of a substance.
Connects atomic scale with practical chemistry at the macro level.
Measurement Units
Atomic Mass:
- Measured in (amu) or dalton.
- Accurate to atom mass.
Molar Mass:
- In grams per mole (g/mol).
- Largens the atomic mass for laboratory measurements.
Role in Chemistry
Atomic mass
- Crucial for comprehending elemental properties.
- Important for atom and molecule structure and behavior.
Molar mass
- Crucial for chemical calculations, including establishing reactant and product amounts.
- Used widely in lab work to prepare solutions and conduct tests.
Calculation Methods
Atomic Mass:
- A weighted average of an element’s natural isotope mass numbers.
- Must know isotopic composition and abundance.
Molar mass
- Occurs by adding the atomic masses of all atoms in a formula or molecule.
- Simple computation using the compound’s molecular or empirical formula.
Difference Between Atomic Mass vs Molar Mass
Summary
Atomic mass and molar mass are fundamental but distinct concepts in chemistry. A precise assessment of each atom’s mass illustrates how elements are structured and their atomic characteristics. It is like locating the notes in a hard melody. Molar mass, however, brings complex atomic details to labs. Imagine how all these notes fit in an orchestral performance. This is crucial for chemical research and applications.
Although they operate on different scales – atomic mass on the micro, and molar mass on the macro – their roles are deeply interconnected. They explain how chemicals act from atoms to chemical processes. Chemistry is precise and useful, and this dualism honors theory and practice’s good fit.
FAQS
1. How do you find molar mass from atomic mass?
Simply convert an element’s atomic mass (amu) to grams per mole (g/mol) to obtain its molar mass. The number stays the same. Sum the atomic masses of all atoms in a compound to find its molar mass.
2. What is the difference between molar mass and mole mass?
The mass of one mole of a substance (given in g/mol) is called molar mass, while mole mass is a less common word that can be misinterpreted.
3. What is the difference between atomic mass, molecular mass, and formula mass?
Atomic mass is one atom’s mass in amu. A molecule’s mass is the total of its atoms’ masses. Ionic chemicals employ formula mass instead of molecular mass.
4. What is the difference between atomic mass and molar mass (as per Quizlet)?
A single atom’s isotopic makeup determines its atomic mass, generally in amu. Molar mass is the mass of one mole of a substance (in g/mol), expanded from atomic mass.
5. Why is molar mass the same as atomic mass?
Molar and atomic masses have the same number but distinct units. Since molar mass is a scale-up of atomic mass to account for one mole of particles, the numerical value is the same but the unit is g/mol.
6. Is molar mass just mass?
Molar mass is the mass of one mole. It is the mass of Avogadro’s number of particles (atoms, molecules, or formula units) of that substance.
- Difference Between Psychopath and Sociopath - April 2, 2024
- Difference Between IQ and EQ - April 2, 2024
- Difference Between Good Carbs and Bad Carbs - April 2, 2024
Search DifferenceBetween.net :
 Email This Post
: If you like this article or our site. Please spread the word. Share it with your friends/family.
Email This Post
: If you like this article or our site. Please spread the word. Share it with your friends/family.
3 Comments
Leave a Response
References :
[0]Image credit: https://www.canva.com/photos/MADmTJD13cs--atomic-level-close-up-of-the-trypsin-digestive-enzyme-surface-view-of-the-protein-surface/
[1]Image credit: https://www.canva.com/photos/MAFo8QzjszY-periodic-table-of-chemical-elements-top-view/


I beg to disagree. Atomic mass of an element is the weighted average mass of its isotopes. It is basically the same as the mass number (the number of protons + number of neutrons in an element). It is also similar to molar mass (mass of one mole of the substance). Molecular Weight, on the other hand, is not similar to atomic mass, neither it is similar to mass number nor molar mass. Molecular weight is the mass of the substance in amu that corresponds to the chemical formula of the substance. And to calculate molar mass, you need to use the atomic masses of all the elements that make up the substance(molecule).
These definitions are taken from Chemistry Reader 2A by Andreas Toupadakis.
My bad. I meant to say molar mass is the same as molecular mass. However, it is different from atomic mass and mass number.
One thing ,in case of monoatomic metal 1 molecule will be equal to 1 atom of the element.for ex sodium a molecular mass =23g and atomic mass is 23amu
So ,when to consider atomic mass or molecular mass in chemical reaction