Difference Between Evaporation and Boiling
Evaporation vs. Boiling Article
What is Evaporation?
Evaporation is a process where liquid turn into vapor. Example is “water evaporated from the soil”
What is Boiling?
Boiling means rapid vaporization of any liquid. It happens when a liquid is heated to its boiling point. The boiling occurs in three different stages such as nucleate boiling, transition boiling and film boiling. There are no stages for evaporation.
Evaporation vs. Boiling
Boiling occurs when the temperature of the liquid is greater than the boiling point of the substance. Evaporation can occur at any temperature. It occurs as long as the substance remains liquid at a particular temperature.
According to Greg Bradburn, evaporation occurs when there is an increased energy present and occurs rapidly. It occurs from the bottom of the container when allowed to boil. The bubbles form at the bottom of the container and then rise on top of the container. In boiling, bubbles do not form at the bottom and rise to the surface. Evaporation occurs at room temperature and therefore, occurs at a slower rate when compared to boiling.
In boiling, there is formation of bubbles as it is a complex physical process and these bubbles are formed on a heated liquid. There is cavitation and acoustic effects can be seen as well.
There is no such bubbles formed in evaporation and there is no cavitation and acoustic effect present in evaporation.
The microscopic difference between evaporation and boiling is as follows:
In boiling, the motion of particles is increased and this force separates the particles apart from each other. The temperature is uniform and the boiling also occurs throughout.
In evaporation the movement of the particles is not the same. Few particles move at slower speed and few particles move at an higher speed. The surface particles are held in place by the particles beneath the surface layer and the particles in the middle layer is held by the forces acting on the sides of the container. The particles on the surface can break easily from the liquid.
| BOILING | EVAPORATION | |
|---|---|---|
| Definition of these two terms | Boiling is vaporisation of liquid into gas due to continuous heating. Most of the time it is not a natural process. | Evaporation on the other hand is a natural process and it is similar to boiling , but the liquid form changes into gaseous form when there is a increase in either temperature or pressure or both. |
| Occurrance | Large mass | only on surface |
| Amount temperature involved | Boiling happens when the temperature reaches to the boiling point. | In Evaporation the temperature does not need to reach to boiling point |
| Bubble Formation | Bubble formation is visible | Bubbles do not form in evaporation |
| Energy | Usually external source of energy is needed for boiling to happen. | Most of the time energy for evaporation is supplied by the atmosphere or surrounding. |
| Length of the process | Rapid Process | Slow process compared to boiling |
SUMMARY OF BOILING AND EVAPORATION:
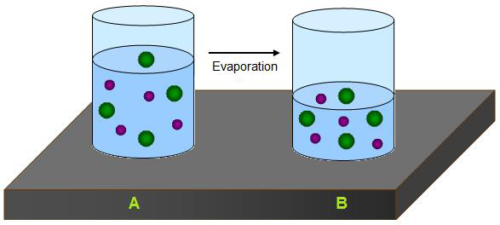
1. Evaporation occurs on the surface of the liquid whereas boiling occurs at the entire length of liquid.
2. Boiling occurs rapidly whereas evaporation occurs slowly.
3. Evaporation occurs at any temperature whereas boiling occurs at a specific temperature.
4. The motion of particles is fast in boiling whereas in evaporation few particles move slowly and few at a faster rate.
5. There is formation of bubbles in boiling, but bubbles are not seen in evaporation.
- Difference Between Cell Wall and Cell Membrane - December 19, 2009
- Difference Between Evaporation and Boiling - December 19, 2009
- Difference Between OCD and OCPD - December 19, 2009
Search DifferenceBetween.net :
35 Comments
Leave a Response
References :
[0]By Rebecca Calhoun [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons

please please please we want a summary!! write another one but shorter please!!
i am alien from 2017 but im here in 2014
Wow awesome
I want some question about these experiments so please help me
It is very nice but it is very much long. Please make another but shorter
:-)It is very nice and it help me in my project work.
Why are you guys pointing out all the negatives. The author does have a summary. And it even is in bullet points. So quit showing so much attitude.
Cool
Bro chill!!!! We don’t need to worry about the whole world…
If we atually consider the facts and points given they have proved themselves right and it has personally helped me a lo
So, Nice it help me a lot ………………. . Thankssssss
Helped me a lot!!!!
Yes
Difference between boiling and evaporation
It help alot and in my assignment
Lol, im a alien lol …
Lol I’m also here in 2018
Explain the phenomnon evaporation boiling
Should give some examples..
Thankss… tomorrow is my exam
Ok
Thanks for the details answer. I was searching for the answer as i had faced this question in an interview but i was failed to answer that.
Good information. Thanks to the website. It really helped me a lot.
It’s nice but make it short
Thankss i love it it helps me sôô much
Thanku so much my studies to so misc help
thank u much I’ve helped me alot
thanks u’ve helped me very much
It helped me a lot through every subject in this this chapter.
I love this ♥️ website
It really helped
It is too long and also make it simpler
Still working on it but try to make points short and brief
Wow
This is great.
Thanks for this.
This really helped me.