Difference Between Ionic and Covalent Compounds
Introduction
In chemistry, we discover a wide universe of chemicals that make up everything. Chemical compounds, amazing combinations of elements in many forms, are at the center of this intricacy. Ionic and covalent chemicals are two major categories with distinct properties. You may ask why these matters. Understanding these molecules is not just academic—it is vital for health, technology, and environmental research, affecting everything from pharmaceuticals to electronics to air.
The nature of chemical bonds, which entail the transfer or exchange of electrons to establish stability, characterizes chemical compounds. Ionic and covalent bonding are the two main categories for this activity, which is essential to material characteristics. Ionic bonding is the process by which electrons are moved from one atom to another, creating ions with opposite charges. In contrast, electron sharing occurs during covalent interaction between atoms. In order to assure correctness and clarity in the communication of scientific concepts, this discussion seeks to offer a thorough grasp of these two bonding kinds, free of metaphorical language and colloquialisms.
What is an Ionic Compound?
Definition
Ions are created when atoms from various elements exchange electrons to produce ionic compounds. These charged particles, called ions, work similarly to magnets in that they create an electrical attraction. In this process, a positively charged cation and a negatively charged anion are formed when a metal atom donates one or more electrons to a non-metal atom. An ionic connection is created when these oppositely charged ions are attracted to one another electrostatically.
Characteristics of Ionic Bonds
- Strong and stable ionic bonds are produced by electrostatic forces. Because of their strength, ionic compounds have high melting and boiling points.
- Conductivity of Electricity: Ionic compounds can be distinguished from other materials by their easy conductivity of electricity when melted or dissolved in water.
- Chemicals with ions dissolve in water. Ionic chemicals have favourable interactions with water molecules, which allows them to dissolve easily in aqueous solutions.
- Ionic compounds exhibit solidification through the formation of organised, repeating patterns known as crystal lattices in all three dimensions.
Examples of Ionic Compounds
Table salt (NaCl) is a basic ingredient in our kitchen. Sodium (Na), an ionic molecule, forms a strong and essential bond with chlorine (Cl) by giving it an electron. Another well-liked de-icer for roads is CaCl2. The electron-donating property of calcium demonstrates the versatility and abundance of ionic compounds in both nature and industry.
These examples show that ionic chemicals are important in everything from food to technology. Understanding these substances illuminates the tiny interactions that affect our macroscopic environment as we study chemistry.
What is a Covalent Compound?
Further within the molecular ballet, covalent compounds become another intriguing chemical character. Atoms share electrons to produce these compounds, forming a mutually beneficial alliance. This sharing lets them establish a noble gas electron configuration, achieving chemical stability.
Definition
Covalent compounds are bonds formed when two atoms share their electrons to reach a stable electron configuration. Sharing electrons is essential to form covalent bonds in non metal atoms with similar electro-negativities to reach a balanced distribution of electrons.
Features of Covalent Bonds
- Molecular structure:
How atoms share electrons and how electrons are distributed between them determines the specific shapes and sizes of molecules.
- Physical properties:
Covalent compounds have low melting and boiling points compared to ionic compounds and this results in lower force in holding the molecules together.
- Variable states:
Covalent compounds can be found in gases, liquids or even solids at room temperature.
- Covalent compounds do not conduct electricity as they don’t have free electrons.
Covalent Compound Examples
Water (H2O), carbon dioxide (CO2), and methane (CH4) are among the many covalent molecules. Water, the universal solvent, carbon dioxide, a major role in photosynthesis and the greenhouse effect, and methane, a basic but potent greenhouse gas, demonstrate covalent bonding’s variations.
This little explanation has demonstrated how atoms combine to form covalent compounds through sharing electrons.
Possible Similarities Between Ionic and Covalent Compounds:
Both are types of chemical compounds:
Molecules that are ionic or covalent make up every matter. They are the building blocks of all living things, from simple molecules to complicated ones.
Both involve the sharing or transferring of electrons:
In order for ionic molecules to be stable, they need full electron transfer. For covalent bonds to form, both full electron transfer and mutual electron sharing are necessary. This transference of electrons controls their chemical properties and how they respond with other chemicals.
Aim for stability:
The shared goal of both ionic and covalent bonds is to keep matter stable. Following the octet rule, atoms join together to fill their outer electron shells. This makes for a more energetically favorable shape.
Ionic and covalent bonds help make molecules and compounds, which are the building blocks of many things. Ionic compounds in rocks, gases in the air, and biological molecules in living things are all examples of these substances.
Ionic and covalent combinations have different melting points, boiling points, solubility, and electrical conductivities because of the links that hold them together. This shows how sharing and exchanging electrons can change how drugs act in different situations.
By understanding these similarities, we can better understand the complicated compounds that shape every matter.
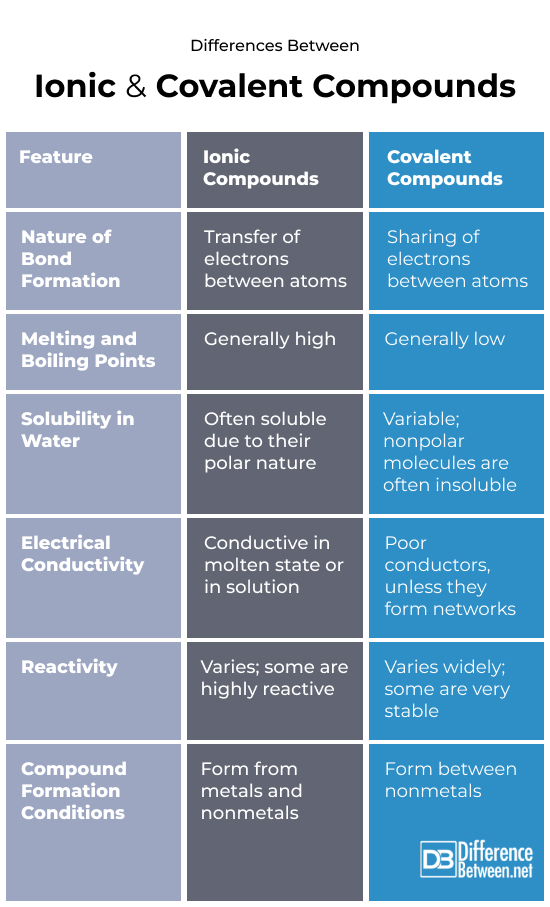
Differences Between Ionic and Covalent Compounds
The reactions among atoms that makes up our world shows us the differences between ionic and covalent substances. These differences set each relationship apart and affect how they act and connect with the rest of the world. An outline:
Nature of bond formation
Ionic: When electrons are moved from one atom to another, ions with opposing charges are created, which is how ionic bonds are formed. Positively charged cations and negatively charged anions are produced as a result of this transfer. The ionic link is formed between these ions by electrostatic attraction, which also adds to the ionic compound’s stability.
Covalent: When atoms share electrons to form a stable electron configuration, covalent bonds are created. The atoms are able to stabilize to a degree that is similar to noble gases because to this sharing of electrons. Covalent bonding creates a stable molecule structure by involving the mutual distribution of electrons between atoms, most often nonmetals. Physical Properties:
Melting and Boiling Points:
- Ionic compounds have high melting and boiling points, which means they need a lot of heat to break apart.
- It is easier for covalent molecules to break apart because their melting and boiling points are lower.
Solubility in Water:
- Ionic chemicals are like sugar in tea in how easily they dissolve in water.
- Chemicals that have covalent bonds, like oil, might not want to be near water.
Electrical Conductivity:
- Ionic objects conduct electricity like live lines when they melt or dissolve because the ions are free to move around.
- Covalent molecules can not conduct electricity because they do not have any free ions.
Chemical Properties:
Reactivity:
- Ionic compounds are very reactive and start chemical reactions when they come into contact with molecules that have opposite charges.
- When two molecules share electrons in covalent compounds, they can be very unstable or very stable.
Conditions for Compound Formation:
- Ionic compounds form under conditions where atoms can easily transfer electrons, typically between metals and nonmetals.
- Covalent compounds form when atoms, usually nonmetals, are close enough to share electrons, requiring a balance of electronegativities.
These differences between covalent and ionic molecules in chemistry show how atoms become more stable. By sharing or moving electrons, each type of chemical adds to the beauty and complexity of matter.
Differences Between Ionic and Covalent Compounds
Summary
We discovered the distinctions between ionic and covalent bonds by studying chemical molecules. Electron transfer between metals and nonmetals creates ionic compounds, which have high freezing and boiling temperatures, water solubility, and solution electrical conductivity. Covalent compounds develop when nonmetals share electrons. These have low melting and boiling temperatures, solubility fluctuations, and poor electricity conductivity.
Discovery of these inequalities is fascinating and practical. Molecular chemical links affect quality and utility in material development and pharmaceutical production. Ionic or covalent state can help scientists and engineers solve some of the world’s toughest problems by predicting product behavior in different environments.
To conclude, ionic and covalent molecules alter how we think about chemical interactions, build technology, and cure diseases. Although we are conducting molecular research, these discrepancies are essential for maximizing chemical utilization in science and business.
FAQs
What is the difference between an ionic bond and an ionic compound?
An ionic bond is the electrostatic attraction between positively and negatively charged ions, while an ionic compound is a substance formed by the combination of atoms connected by ionic bonds, typically consisting of a metal and a nonmetal.
What is an example of a covalent bond and an ionic bond?
Covalent bond example: H₂O (water), where oxygen and hydrogen share electrons.
Ionic bond example: NaCl (table salt), where sodium donates an electron to chlorine, forming positive and negative ions that attract each other.
What is the difference in electronegativity?
- The difference in electronegativity refers to the ability of an atom to attract shared electrons in a chemical bond. In ionic bonds, this difference is typically greater, leading to the transfer of electrons. In covalent bonds, the difference is smaller, resulting in shared electrons.
Which bond is stronger, ionic or covalent?
It depends on the context. Generally, covalent bonds are considered stronger in individual molecules, but ionic compounds can form strong crystalline lattices. The strength also varies with the environment (e.g., covalent bonds in organic molecules can be very strong).
What are 5 examples of ionic bonds?
- NaCl (Sodium chloride)
- CaCl₂ (Calcium chloride)
- KBr (Potassium bromide)
- MgO (Magnesium oxide)
- Na₂O (Sodium oxide)
How do you easily tell the difference between ionic and covalent?
Look at the elements involved: metals combined with nonmetals typically form ionic bonds, while nonmetals combined with other nonmetals form covalent bonds. Also, consider electronegativity differences: large differences suggest ionic bonds.
What is the easy difference between ionic and covalent bond?
Ionic bonds involve the transfer of electrons from one atom to another, forming ions. Covalent bonds involve the sharing of electrons between atoms.
How do you identify a covalent bond?
Identify if both elements in the bond are nonmetals. If so, and they share electrons for stability, the bond is covalent. The presence of molecular compounds, especially organic ones, typically indicates covalent bonding.
- Difference Between Suicide and Euthanasia - May 22, 2024
- Difference Between Vitamin D and Vitamin D3 - May 21, 2024
- Difference Between Running Shoes and Walking Shoes - April 30, 2024
Search DifferenceBetween.net :
11 Comments
Leave a Response
References :
[0]Butts, B., & Smith, R. (1987). HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds. Research in Science Education, 17, 192-201.
[1]Alberts, B., Bray, D., Hopkin, K., Johnson, A. D., Lewis, J., Raff, M., ... & Walter, P. (2015). Essential cell biology. Garland Science. (pp. 39-49).
[2]Butts, B., & Smith, R. (1987). HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds. Research in Science Education, 17, 192-201.
[3]Butts, B., & Smith, R. (1987). HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds. Research in Science Education, 17, 192-201.
[4]Alberts, B., Bray, D., Hopkin, K., Johnson, A. D., Lewis, J., Raff, M., ... & Walter, P. (2015). Essential cell biology. Garland Science. (pp. 39-49).
[5]Alberts, B., Bray, D., Hopkin, K., Johnson, A. D., Lewis, J., Raff, M., ... & Walter, P. (2015). Essential cell biology. Garland Science. (pp. 39-49).
[6]Alberts, B., Bray, D., Hopkin, K., Johnson, A. D., Lewis, J., Raff, M., ... & Walter, P. (2015). Essential cell biology. Garland Science. (pp. 39-49).
[7]Alberts, B., Bray, D., Hopkin, K., Johnson, A. D., Lewis, J., Raff, M., ... & Walter, P. (2015). Essential cell biology. Garland Science. (pp. 39-49).
[8]Image credit: https://www.canva.com/photos/MADq34Stdeg-f025-0181/
[9]Image credit: https://www.canva.com/photos/MAEa_ZlmBYs-amino-acid-molecule-compound-3d-rendering/

I remember hearing about molecular symmetry being a useful indicator of whether a molecule was ionic or covalent. Is that worth mentioning in this article?
No
Maybe. Good thought Thomas!! 🙂
I am confused before reading but I am perfect after reading this statement .THANKU for your kind
information
that was the most useless thing in my life
Wonderful . Thank you for your useful information . I was really confused between them before reading your article but I am perfect after these information ….. thank you ☺☺
IF THERE IS ANYONE FROM BCC HS, COMMENT BABY
I want diffrence betweean ionic compound and polar covalent compounds
Thanks for this explanation . But i m not confused
This information will realy help me a lot with my assignment en please do keep me updated
Thank you so much