Difference Between Hydrolysis and Dehydration Synthesis
• Categorized under Health | Difference Between Hydrolysis and Dehydration Synthesis
The Difference between Hydrolysis and Dehydration Synthesis
Biosynthesis is essential in all living organisms – it is the integration of life. This is organic processes, which involve simple compounds to be modified, joined together or converted into other compounds to form macromolecules. There are two processes that play vital roles in biosynthesis. These are Hydrolysis and Dehydration Synthesis.
Hydrolysis and Dehydration Synthesis both deal with water and other molecules, but in very different ways. Both have a reverse reaction in relation to each other and vice versa. In biology, these processes involve the formation of Polymers, these are molecules covalently linked together. These are formed when water is removed from a chemical equation then monomers (small molecules) bond together. In order to break the bonds, water must be added to the equation. To further understand this, detailed information regarding the difference between hydrolysis and dehydration synthesis is discussed below.
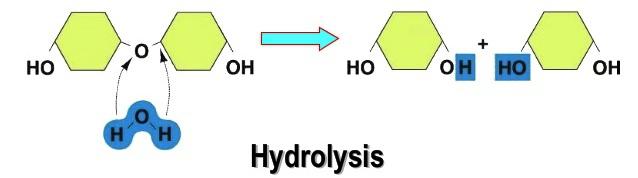
Hydrolysis
Hydrolysis means separating with the use of water. It comes from the Greek word “hydro” which means water, and “lysis” which means separation. When water is added to a molecule, it breaks the H2O bond into H and OH forming separate molecules.
In Chemistry, Hydrolysis is a chemical reaction with water, in which a macromolecule is separated into smaller molecules. On the other hand, in Biology, this process involves water to split polymers into monomers. The bottom line is Hydrolysis occurs when water is added to the equation to break it down or separate it.
In our bodies, Hydrolysis is the main process to release energy. When we eat food, it is digested or broken down into substances so the body can absorb it and convert it to energy. Foods, having complex molecules are broken down into simple molecules. When energy is needed for biosynthesis, ATP is hydrolyzed and stored energy is released for utilization.
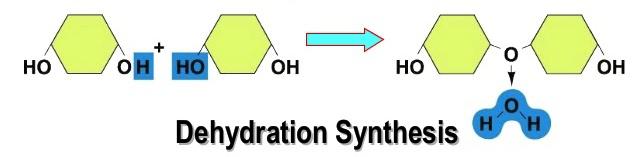
Dehydration Synthesis
Dehydration means to take away water, and synthesis means to build or create something. Hence, Dehydration Synthesis is defined as taking away water to build something. This process happens by removing one molecule of –OH (hydroxyl group) and one molecule of -H to form H2O or water. This results in covalently joining two monomers (small molecules) to form a polymer (larger molecule).
Dehydration Synthesis uses condensation in the process and when this continues for a long period of time, a long and complex chain is formed, just like the ones in polysaccharides. It is also is responsible for storing excess glucose molecules as much as larger polysaccharides like starch and glycogen.
Examples of Hydrolysis and Dehydration Synthesis
Hydrolysis and Dehydration Synthesis work the same way with proteins, carbohydrates, nucleic acids and lipids. As mentioned earlier, in the process of Hydrolysis – when water is added, it separates the bond between oxygen and hydrogen and reforms into two separate hydroxyls. In contrast, in the process of Dehydration Synthesis you have a hydroxyl on each side, so if oxygen and two hydrogens are taken out and bind the remaining oxygen to the remaining hydrogen to form a polymer.
|
Carbohydrates |
Hydrolysis |
Dehydration Synthesis |
| Disaccharide + H2o = Monosaccharide + Monosaccharide
Sucrose + H20 = Fructose and Glucose |
Monosaccharide + Monosaccharide = H2O + Disaccharide
Glycosidic Linkage: two carbohydrates are joined together when an H from one carbohydrate and an OH from the other is taken out and forms H2O |
|
|
Lipids |
Lipid + 3H2O = 1 Glycerol + 3 Fatty Acids | 1 Glycerol + 3 Fatty Acids = Lipid + 3H2O |
|
Protein |
Dipeptide + H2O = 2 Amino Acids | Amino Acid +Amino Acids = Dipeptide + H2O
A peptide bond is a result when the removal of H atom from one amino acid and an OH from the other. |
|
Nucleic Acid |
Nucleic Acid + H2O = 10 Nucleotide | 10 Nucleotide = Nucleic Acid + H2O |
- The Difference between Pemphigus and Pemphigoid - July 9, 2015
- The Difference between Flu and Influenza - July 9, 2015
- The Difference Between a Wound and an Ulcer - June 22, 2015
Sharing is caring!
Search DifferenceBetween.net :
Cite
APA 7
Sison, J. (2015, April 15). Difference Between Hydrolysis and Dehydration Synthesis. Difference Between Similar Terms and Objects. http://www.differencebetween.net/science/health/difference-between-hydrolysis-and-dehydration-synthesis/.
MLA 8
Sison, Jade. "Difference Between Hydrolysis and Dehydration Synthesis." Difference Between Similar Terms and Objects, 15 April, 2015, http://www.differencebetween.net/science/health/difference-between-hydrolysis-and-dehydration-synthesis/.
1 Comment
Leave a Response
Written by : Jade Sison. and updated on 2015, April 15
References :
[0]https://sites.google.com/site/biologymolecules/dehydration-sythesis-vs-hydrolysis
[1]http://www.ask.com/science/difference-between-dehydration-synthesis-hydrolysis-c5fc3ec35aab7049
See more about : Metabolism

You should not be posting wrong information like the way you have here. Students do not know what is correct or not, and are using your site form reference.
“Dehydration synthesis, on the other hand, is the opposite of hydrolysis. It occurs when two molecules join together to form water such as bonding oxygen with hydrogen which produces water” this is summarily wrong.