The Difference between Effusion and Diffusion
If you look at the Periodic Table of Elements, you can see numerous substances that make up the environment. It is so amazing to know that everything is made up of small identifiable unit known as molecules. These molecules are made up of atoms, which are held by chemical bonds. These bonds are result of exchanges or sharing of electrons (negatively or positively charge subatomic particles) among atoms.
When it comes to gases, their molecules move in a definite manner. In the 1800’s, Kinetic Molecular Theory was formulated by two scientists: James Maxwell and Ludwig Boltzman. They explained how gases behave and came out with four postulates that recount the theory. The following are:
- The molecules of gases are in constant motion and the collision between gas molecules and the container walls causes pressure.
- The particles of gas do not interact with each other. There is a neither repulsive nor attractive force.
- The Kelvin temperature is directly proportional to the average kinetic energy.
- The particles of gases are so small compared to the volume they occupy, hence the particles are considered to have no volume.
In addition to this, Thomas Graham, a Scottish chemist formalized how gases move about. Thus, The Graham Law governs the effusion and diffusion phenomenon. However, take note that these terms are not only confined in chemistry, but these are also widely used in many sciences such as physics and biology. When it comes to chemistry, Effusion and Diffusion are some of the phenomena that gases and liquids move, in which most people get confused with because of the similar sounding names. However, they are entirely different from each other.
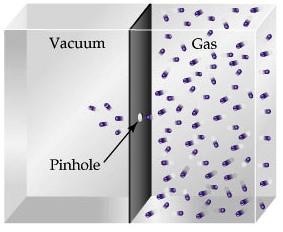
Effusion is described as the movement or escape of gas molecules through a pinhole into a vacuum.
Basing on the theory discussed above, a lighter gas effuses at a greater speed compared to heavier ones because the molecules collide with the hole resulting in more particles escaping through in unit time.
This is made quantitative by the Graham’s Law – the molecular movement is inversely proportional to the square root of its molar mass.
A perfect example of effusion is the phenomenon that occurs with inflated balloons. Have you ever noticed how a balloon deflates over a period of time? Well, that is because the air inside the balloon escapes through a pinhole or a small hole to the environment.
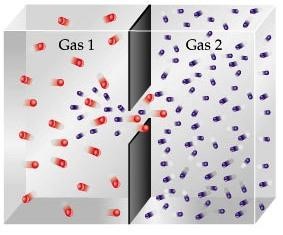
Diffusion comes from the Latin word “diffundere” which means to “spread out”. In chemistry, Diffusion is described as the gradual mixing of gases. It is the movement of one gas through another by thermal random motion causing the molecules to collide with each other and exchanging molecular energy in between them.
It is a gradual process, which causes the molecules to move from an area of higher concentration to an area lower concentration. This continuously occurs and only stops when the molecules are evenly spread out.
The easiest way to understand diffusion is when a bottle of perfume is opened. The fragrance travels and spread out into the surrounding air. The gradual fragrance mixing with the air is observed when a person near the opened perfume bottle smells it first and eventually the person farthest to the source smells it later. Same thing happens when a certain individual farts, the noxious smell diffuses with the air, and sooner or later people will smell it. So, avoid farting in public places.
Final Thought!
Diffusion and Effusion play a vital role in our everyday lives. In fact, diffusion is a common process that occurs inside the body. It is the process that happens between the exchanges of nutrients, energy and oxygen within our systems. It is quite informative to know how elements move about and know the exact difference between effusion and diffusion.
- The Difference between Pemphigus and Pemphigoid - July 9, 2015
- The Difference between Flu and Influenza - July 9, 2015
- The Difference Between a Wound and an Ulcer - June 22, 2015
Search DifferenceBetween.net :
Leave a Response
References :
[0]http://mail.colonial.net/~cricket/chemistry/assets/flipcharts/grahammcc.pdf