Difference Between Hydrophilic and Hydrophobic
Hydrophilic vs. Hydrophobic
Solvents, mixtures, compounds, and particles are just some of the components of a chemist’s life. Studies involving the observance of molecule behavior in any given state or environment may seem to be one of the most brain-whacking jobs for those with little background in chemistry and related sciences, but these are very helpful in coming up with the latest products and developments in various industries.
Chemists, biologists, and other individuals pursuing a career in the field of science, start their career by attaining the necessary training from universities and colleges. When they decide to have a career related to biochemistry, their education starts with lessons that give them a deeper understanding of molecular activities and behavior.
That being said, it is safe to assume that the basic courses offered during their first year of college include an evaluation of the hydrophobic and hydrophilic nature of molecules and other particles.
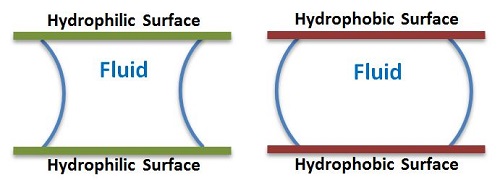
The word “hydro-” means “water.” Thus, studying hydrophobic and hydrophilic molecules concerns the solubility and other properties of particles as they interact with water. The term “–phobic,” originating from “phobia,” would translate into “fearful of (water).” Hydrophobic molecules and particles, therefore, can be defined as those that do not mix with water – they repel it. On the other hand, hydrophilic molecules are those that interact well with H2O.
In other words, the distinction between hydrophobic and hydrophilic molecules is drawn by observance of the hydrophobic particles’ repellency of water and hydrophilic molecules’ attraction to water.
In a laboratory experiment, for example, one can observe that there are particular solubles that dissolve in water, and others that do not. Crushed and powdered makeup, for example, may be able to dissolve in a glass full of cooking oil, but not in a glass full of water. Salt, on the other hand, is easily absorbed by water, but it may not dissolve in oil.
Crushed and powdered makeup, therefore, can be seen as hydrophobic particles. Meanwhile, students can arrive at the conclusion that the molecules of salt are hydrophilic. Salt can keep a strong affinity in water, which can absorb and dissolve it. On the other hand, the oil-based makeup contains molecules that repel and refuse to combine with the molecules of water.
Aside from laboratory experiments, this molecular behavior in reference to the hydrophobic and hydrophilic nature is also observed when biologists look into the permeability of cell membranes. Note that several particles may enter and exit the cell through the membrane, which is made of lipid bilayers and proteins.
When the particles are hydrophobic, a simple passive diffusion occurs, which means that the molecule does not need the exertion of energy to enter or exit the cell. This is because the cell membrane comes with hydrophobic components that match the molecules.
On the other hand, hydrophilic particles may need protein carriers for facilitated diffusion. This is because the components of the molecules reject those of the cell membrane.
To get a clearer understanding of this, picture a glass of water and a glass of cooking oil. When water is added to the oil, there is repulsion between the molecules. But when one puts water into water and oil into oil, no reaction will be observed.
Organic chemistry provides an explanation for this phenomenon. Note that water contains polar molecules; it therefore follows that polar substances and particles get absorbed or attracted by H2O. Hydrophilic molecules are known to be polar and ionic – they have positive and negative charges, which can attract water molecules. Conversely, hydrophobic particles are known to be non-polar.
Summary:
1.Hydrophilic means water loving; hydrophobic means resistant to water.
2.Hydrophilic molecules get absorbed or dissolved in water, while hydrophobic molecules only dissolve in oil-based substances.
3.Hydrophilic molecules require facilitated diffusion, while hydrophobic molecules are suitable for passive diffusion in cellular activities.
4.Hydrophilic molecules are polar and ionic; hydrophobic molecules are non-polar.
- Differences Between Fraternity And Sorority - January 8, 2014
- Differences Between Lucite and Plastic - January 7, 2014
- Differences Between Oil and Butter - January 6, 2014
Search DifferenceBetween.net :
4 Comments
Leave a Response
References :
[0]https://commons.wikimedia.org/wiki/File:Fluid_Between_Hydrophilic_and_Hydrophobic_Plates.JPG

not nice very bad
Hello Priya ge
Not bad
Good basic info