The Difference Between Osmosis and Active Transport
A cell has many requirements in order to grow and replicate, and even cells that aren’t actively growing or replicating require nutrients from the environment to function. Many of the cell’s requirements are molecules that can be found outside the cell, including water, sugars, vitamins and proteins.
The cell membrane has important protective and structural functions, and it acts to keep cellular contents separate from the exterior environment. The lipid bilayer of the cell membrane is composed of phospholipids, which have hydrophobic (oil soluble, “water-fearing”) tails that form a barrier to many solutes and molecules in the environment. This feature of the cell membrane allows the cell internal environment to differ from the external environment, but also acts as a major barrier to taking up certain molecules from the environment and expelling waste.
The lipid bilayer does not pose a problem for all molecules, however. Hydrophobic (or oil soluble), nonpolar molecules can freely diffuse through the cell membrane unimpeded. This class of molecules includes gases such as oxygen (O2), carbon dioxide (CO2), and nitric oxide (NO). Larger hydrophobic organic molecules can also pass through the plasma membrane, including certain hormones (such as estrogen) and vitamins (such as vitamin D). Small, polar molecules (including water) are partially hindered by the lipid bilayer but can still pass through.
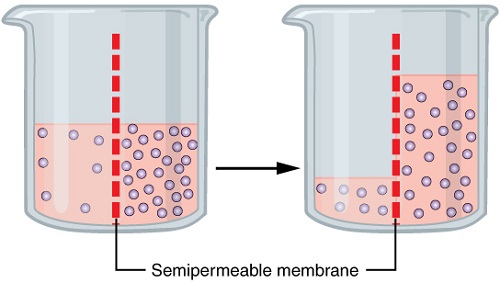
For molecules that can freely pass through the cell’s membrane, whether they travel into or out of the cell depends on their concentration. The tendency of molecules to move according to their concentration gradient (that is from higher concentration to lower concentration) is called diffusion. This means that molecules will flow out of the cell if there are more inside the cell than outside. Likewise, if there are more outside the cell, molecules will flow into the cell until a balance is met. For example, consider a muscle cell. During exercise, the cell converts O2 to CO2. As oxygenated blood enters the muscle, O2 travels from where the concentration is higher (in the blood) to where it is lower (in the muscle cells). At the same time, CO2 travels out of the muscle cells (where it is higher) to the blood (where it is lower). Diffusion does not require energy expenditure. The diffusion of water is given a special name, osmosis.
For larger polar molecules and any charged molecules, entering and leaving the cell is more difficult as they cannot pass through the lipid bilayer. This class of molecules includes ions, sugars, amino acids (the building blocks of proteins) and many more things the cell needs to survive and function. To fix this problem, the cell has transport proteins that allow these molecules to move into and out of the cell. These transport proteins make up 15–30% of the proteins in the cell membrane.
Transport proteins come in several shapes and sizes, but all extend through the lipid bilayer, and each transport protein has a specific type of molecule that it transports. There are carrier proteins (which are also known as transporters or permeases), which bind to a solute or molecule on one side of the membrane and transport it to the other side of the membrane. A second class of transport proteins includes channel proteins. Channel proteins form hydrophilic (“water loving”) openings in the membrane to allow polar or charged molecules to flow through. Both channel proteins and carrier proteins facilitate transport both into and out of the cell.
Molecules can travel through transport proteins from high concentration to lower concentration. This process is called passive transport or facilitated diffusion. It is similar to diffusion of nonpolar molecules or water directly through the lipid bilayer, except that it requires transport proteins.
Sometimes, a cell needs things from the environment that are present in very low concentration outside the cell. Alternatively, a cell may require extremely low concentrations of a certain solute inside the cell. While diffusion would allow the concentrations inside and outside of the cell to move towards equilibrium, a process called active transport helps to concentrate a solute or molecule either inside or outside of the cell. Active transport requires energy expenditure to move a molecule against its concentration gradient. There are two main forms of active transport in eukaryotic cells. The first type consists of ATP-driven pumps. These pumps use ATP hydrolysis to transport a specific class of solute or molecule across the membrane to concentrate it either inside or out of the cell. The second type (called cotransporters) couples transport of one molecule against its concentration gradient (from low to high) with the transport of a second molecule down its concentration gradient (from high to low).
Cells also use active transport to maintain the proper concentration of ions. Ion concentration is very important for the cell’s electrical properties, controlling the amount of water in cells and other important functions of ions. For example, Magnesium ions (MG2+) are very important for many proteins involved in DNA repair and maintenance. Calcium (Ca2+) is also important in many cell processes, and active transport helps maintain a calcium gradient of 1:10,000. Transport of ions across the lipid bilayer depends not only on the concentration gradient, but also on the electrical properties of the membrane, where like charges repel. The sodium-potassium ATPase or Na+-K+ pump maintains a higher concentration of sodium outside of the cell. Almost one third of the cell’s energy requirement is consumed in this endeavor. This huge energy expenditure for the active transport of ions corroborates the importance of maintaining a balance of molecules in proper cell function.
Summary
Osmosis is the passive diffusion of water across the cell membrane and does not require transport proteins. Active transport is the movement of molecules against their concentration gradient (from low to high concentration) or against their electrical gradient (towards a like charge) and requires protein transporters and the added energy, either through ATP hydrolysis or through coupling to the downhill transport of another solute.
- The Difference between Cyclic and Noncyclic Photophosphorylation - June 1, 2016
- The Difference Between Osmosis and Active Transport - May 20, 2016
- Difference Between Microevolution and Macroevolution - September 22, 2011
Search DifferenceBetween.net :
Leave a Response
References :
[0]Molecular Biology of the Cell, Fourth Edition. Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. New York: Garland Science; 2002. ISBN-10: 0-8153-3218-1ISBN-10: 0-8153-4072-9
[1]Principles of Neural Science, Fifth Edition by Eric R. Kandel, James H. Schwartz, Thomas M. Jessell, Steven A. Siegelbaum, A. J. Hudspeth Publisher: McGraw-Hill Education / Medical; 5th edition (October 26, 2012) ISBN-10: 0071390111 ISBN-13: 978-0071390118
[2]https://en.wikipedia.org/wiki/Osmosis