Difference Between Electron Geometry and Molecular Geometry
Chemistry is the study of matter and it deals with the many ways one kind of matter can be changed into other kinds. It is known that all matter is made of from one or more of about one hundred different kinds of atom. All atoms are composed of three fundamental particles – protons, electrons, and neutrons. A molecule consists of a group of two or more atoms held together in a definite geometrical pattern. When two or more atoms are strongly held together to form a molecule, there are chemical bonds between each atom and its close neighbors. The shape of a molecule conveys a wealth of information and the first step to understanding the chemistry of a molecule is to know its geometry.
The molecular geometry simply refers to the three-dimensional arrangement of the atoms that constitute a molecule. The term structure is rather used in a sense to indicate simply the connectivity of the atoms. The shape of a molecule is determined in terms of the distances between the atomic nuclei that are bonded together. The geometry of molecules is determined by the Valence- Shell Electron-Pair Repulsion (VESPR) Theory – a model used to determine a molecule’s general shape based on the number of electron pairs around a central atom. The geometry of a molecule is given either as the electron geometry or the molecular geometry.
What is Electron Geometry?
The term electron geometry refers to the name of the geometry of the electron pair/groups/domains on the central atom, whether they are bonding electrons or non-bonding electrons. Electron pairs are defined as electrons in pairs or bonds, lone pairs, or sometimes a single unpaired electron. Because electrons are always in constant motion and their paths cannot be precisely defined, the arrangement of the electrons in a molecule is described in terms of an electron density distribution. Let’s take an example of methane, the chemical formula of which is CH4. Here, the central atom is carbon with 4 valence electrons and 4 hydrogen share electrons with 1 carbon to form 4 covalent bonds. This means there are a total of 8 electrons around carbon and there are no single bonds, so the number of lone pairs here is 0. It suggests CH4 is tetrahedral geometry.
What is Molecular Geometry?
Molecular geometry is used to determine the shape of a molecule. It simply refers to the three-dimensional arrangement or structure of atoms in a molecule. Understanding the molecular geometry of a compound helps determine the reactivity, polarity, color, phase of matter, and magnetism. The geometry of a molecule is usually described in terms of bond lengths, bond angles, and torsional angles. For small molecules, the molecular formula and a table of standard bond lengths and angles may be all that is required to determine the geometry of the molecule. Unlike electron geometry, it is predicted by considering only electron pairs. Let’s take an example of water (H2O). Here, oxygen (O) is the central atom with 6 valence electrons so it requires 2 more electrons from 2 hydrogen atoms to complete its octet. So there are 4 electron groups arranged in a tetrahedral shape. There are also 2 single bond pairs, so the resulting shape is bent.
Difference Between Electron Geometry and Molecular Geometry
Terminology for Electron Geometry and Molecular Geometry
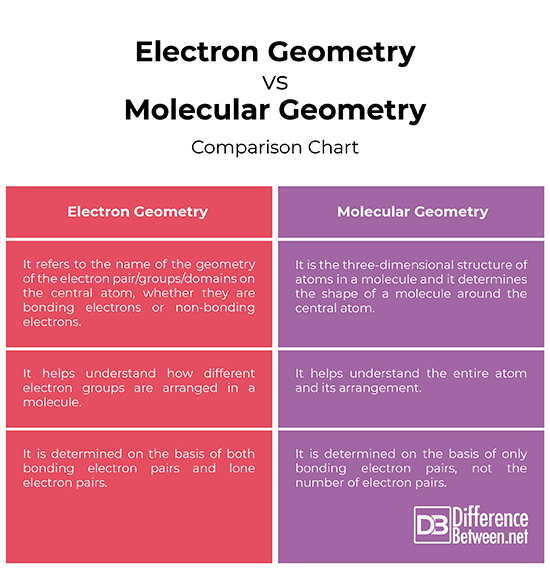
The term electron geometry refers to the name of the geometry of the electron pair/groups/domains on the central atom, whether they are bonding electrons or non-bonding electrons. It helps understand how different electron groups are arranged in a molecule. Molecular geometry, on the other hand, determines the shape of a molecule and it is the three-dimensional structure of atoms in a molecule. It helps understand the entire atom and its arrangement.
Geometry
The geometry of a molecule is determined on the basis of only bonding electron pairs but not the number of electron pairs. It is the three-dimensional shape that a molecule occupies in space. The molecular geometry is also defined as the positions of the atomic nuclei in a molecule. Electron geometry of a molecule, on the other hand, is determined on the basis of both bonding electron pairs and lone electron pairs. The electron geometry can be determined using the VESPR Theory.
Examples of Electron Geometry and Molecular Geometry
One of the many examples of tetrahedral electron geometry is Ammonia (NH3). The central atom here is N and four electron pairs are distributed in the shape of a tetrahedron with only one lone electron pair. Thus, the electron geometry of NH3 is tetrahedral. However, its molecular geometry is trigonal pyramidal because the bond angles are 107 degrees as the hydrogen atoms are repelled by the lone pair of electrons around nitrogen. Similarly, the molecular geometry of water (H2O) is bent because there are 2 single bond pairs.
Electron Geometry vs. Molecular Geometry: Comparison Chart
Summary of Electron Geometry Vs. Molecular Geometry
Both electron geometry and molecular geometry follow the Valence- Shell Electron-Pair Repulsion (VESPR) Model to determine a molecule’s general shape based on the number of electron pairs around a central atom. However, molecular geometry is determined solely on the basis of bonding electron pairs, not the number of electron pair, whereas electron geometry is determined on the basis of both bonding electron pairs and lone electron pairs. When no lone pairs of electrons are present in a molecule, the electron geometry is same as the molecular shape. Like we said, the shape of a molecule says a lot about it and the first step to understanding the chemistry of a molecule is to determine its geometry.
- Difference Between Caucus and Primary - June 18, 2024
- Difference Between PPO and POS - May 30, 2024
- Difference Between RFID and NFC - May 28, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/1/17/O-1057_molecular_geometry.svg/500px-O-1057_molecular_geometry.svg.png
[1]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/e/ea/Electron_configuration_iron.svg/500px-Electron_configuration_iron.svg.png
[2]Gillespie, Ronald and Istvan Hargittai. VSEPR Model of Molecular Geometry. Chelmsford, Massachusetts: Courier Corporation, 2013. Print
[3]Rodger, Alison and Mark Rodger. Molecular Geometry. Oxford: Butterworth-Heinemann, 2014. Print
[4]Bent, Henry. Molecules and the Chemical Bond. Bloomington, Indiana: Trafford Publishing, 2011. Print