Difference Between Endosmosis and Exosmosis
What is Endosmosis?
Definition of Endosmosis:
Endosmosis is the movement of a solvent (usually water) across a semipermeable membrane from a region outside a cell where there is high solvent, low solute, into a cell where this is low solvent, high solute. Endosmosis is a type of osmosis. Osmosis is the passive diffusion of water through a semipermeable membrane along a concentration gradient from where there is a high concentration of water to where there is a low concentration of water.
Tonicity and water potential in Endosmosis:
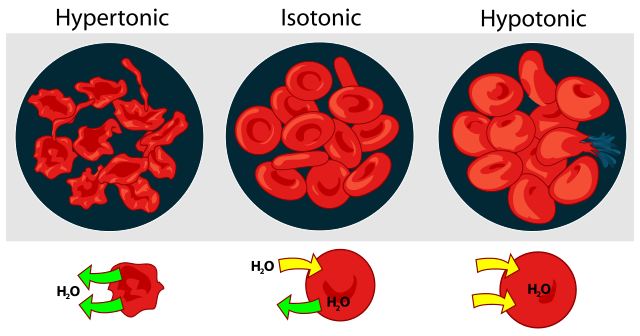
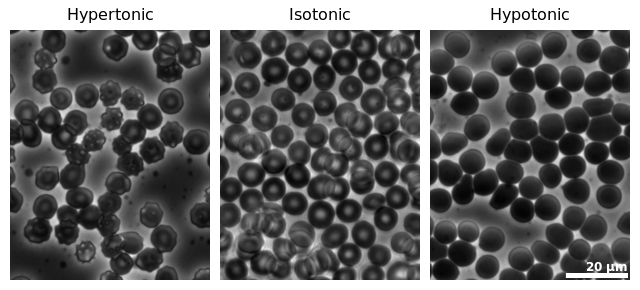
The inside of the cell is hypertonic, that is it has a higher solute concentration than the outside of the cell. The outside of the cell is thus hypotonic, has a lower solute concentration relative to the inside of the cell. The water potential is the tendency for water to move from where there is a high water potential to a low water potential and where there is more solute present there will be lower water potential. Water thus moves by endosmosis into a hypertonic, low water potential cell from a hypotonic, high water potential external environment.
Effects of Endosmosis:
Endosmosis increases the turgor pressure of a cell since the entering water causes the cytoplasm to push against the cell membrane and cell wall (if a plant cell), causing the cell to become turgid. Endosmosis can cause the cell to swell considerably as more and more water enters. If a cell is surrounded by pure water then too much water may enter to the point that the cell swells so much that it bursts, which occurs more often in animal cells than in plant cells since plant cells have a cell wall.
Animal example for Endosmosis:
A freshwater fish may experience changes in ion concentrations in the water in which it lives. The cells of a freshwater fish may at times be hypertonic compared with the external environment. This means that the fish has to osmoregulate and compensate for endosmosis by excreting large amounts of dilute urine.
Human significance in Endosmosis:
Endosmosis is important since it is a process that is needed to keep us alive, but at the same time, if endosmosis occurs too rapidly then a person’s cells can be destroyed and their survival can be jeopardized. For instance, people who drink too much water too quickly can end up with water intoxication or hyponatremia (low sodium).
What is Exosmosis?
Definition of Exosmosis:
Exosmosis is the movement of a solvent (usually water) across a semipermeable membrane from inside a cell where there is high solvent, low solute to outside a cell where this is low solvent, high solute. Exosmosis is also a type of osmosis.
Tonicity and water potential:
The inside of the cell is hypotonic to the outside of the cell. The outside of the cell is thus hypertonic to the inside of the cell. Water thus moves from the high water potential, hypotonic inside of the cell to the low water potential, hypertonic external environment.
Effects of Exosmosis:
When exosmosis occurs water moves out of the cell into the external environment. As this happens the cytoplasm shrinks from water loss. If exosmosis occurs too rapidly and too much water is lost then the cytoplasm will shrink (plasmolysis). If the cytoplasm shrinks too much it could cause the cell to shrink (more commonly occurs in animal cells than plant cells).
Animal example for Exosmosis:
A saltwater fish may experience changes in ion concentrations in the water in which it lives. The cells of a saltwater fish may be hypotonic compared with the external environment. This means that the fish has to osmoregulate and compensate for exosmosis by drinking sea water and producing a small amount of urine.
Human significance of Exosmosis:
Too much exosmosis can lead to dehydration as water is lost from our cells. Severe dehydration can lead to death.
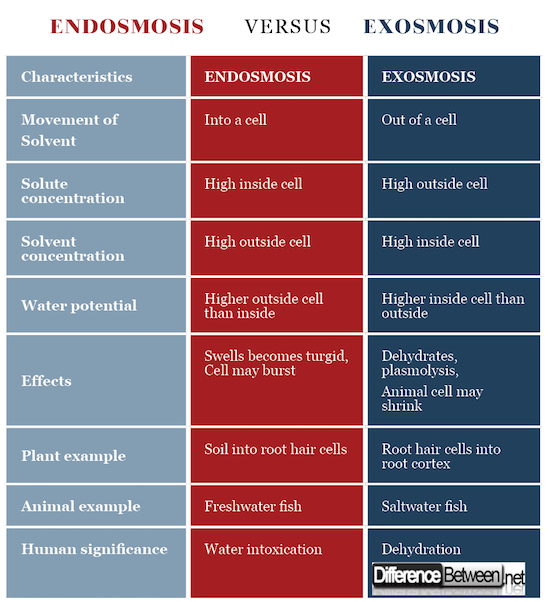
Difference between Endosmosis and Exosmosis
-
Movement of solvent
In endosmosis the solvent moves into a cell while in exosmosis the solvent moves out of a cell.
-
Solute concentration
In endosmosis the higher solute concentration is found inside the cell while in exosmosis the higher solute concentration is found outside the cell.
-
Solvent concentration
In endosmosis the higher solvent concentration is found outside the cell while in exosmosis the higher solvent concentration is found inside the cell.
-
Water potential
Water potential in endosmosis is higher outside the cell then inside, while water potential in exosmosis is higher inside the cell than outside the cell.
-
Effects
In endosmosis the cell becomes swollen and turgid, and may burst (occurs more often in animal cells).
In exosmosis the cytoplasm shrinks and the cell may shrink (occurs more often in animal cells).
-
Plant example
Water moves by endosmosis into root hair cells from the soil while water moves out of root hair cells by exosmosis into the cortex.
-
Animal example
Freshwater fish undergo endosmosis while saltwater fish undergo exosmosis.
-
Human significance
Humans can suffer water intoxication if there is too much endosmosis while they can suffer dehydration if there is too much exosmosis.
Table comparing the difference between Endosmosis and Exosmosis
Summary of Endosmosis Vs. Exosmosis
- Endosmosis and exosmosis are both types of osmosis, but endosmosis is the movement of water into a cell while exosmosis is the movement of water out of a cell.
- Endosmosis occurs when the inside of a cell is hypertonic and has a low water potential, while exosmosis occurs when the inside of a cell is hypotonic and has a high water potential.
- Too much endosmosis or exosmosis can be dangerous.
- Endosmosis and exosmosis have to be carefully regulated for cells to remain alive.
- Difference Between Rumination and Regurgitation - June 13, 2024
- Difference Between Pyelectasis and Hydronephrosis - June 4, 2024
- Difference Between Cellulitis and Erysipelas - June 1, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Ballantyne, Coco. “Strange but True: Drinking Too Much Water Can Kill.” Scientific American. Scientific American, 2018, https://www.scientificamerican.com/article/strange-but-true-drinking-too-much-water-can-kill/
[1]Encyclopedia Britannica. “Osmosis.” Science. Encyclopedia Britannica, 2018, https://www.britannica.com/science/osmosis
[2]Kilgour, O.F.G. Mastering Biology. London: MacMillan Press, 1982. Print.
[3]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/7/76/Osmotic_pressure_on_blood_cells_diagram.svg/640px-Osmotic_pressure_on_blood_cells_diagram.svg.png
[4]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/Human_Erythrocytes_OsmoticPressure_PhaseContrast_Plain.svg/640px-Human_Erythrocytes_OsmoticPressure_PhaseContrast_Plain.svg.png