Difference Between Molarity and Molality
What is molarity?
Molarity can be defined as the number of moles of a substance (known as the solute) that is dissolved in precisely 1 liter of a solution (solvent and solute combined).
The formula for calculating molarity is therefore as follows:
M = mole solute / L solution
Molarity is also commonly referred to as molar concentration. Therefore a measure of molar concentration based on the volume of liquid that a substance is dissolved in. It is important to realize that the volume is in liters so you may have to convert first if you have volume in ml for example.
To prepare a molar concentration one adds a known quantity of solute to a volumetric flask then fills up the flask with liquid until the 1 liter mark is achieved.
For example: one can make up a certain molar concentration of sugar. The weight of the sugar first has to be converted to moles and then the water is added until 1 liter is reached.
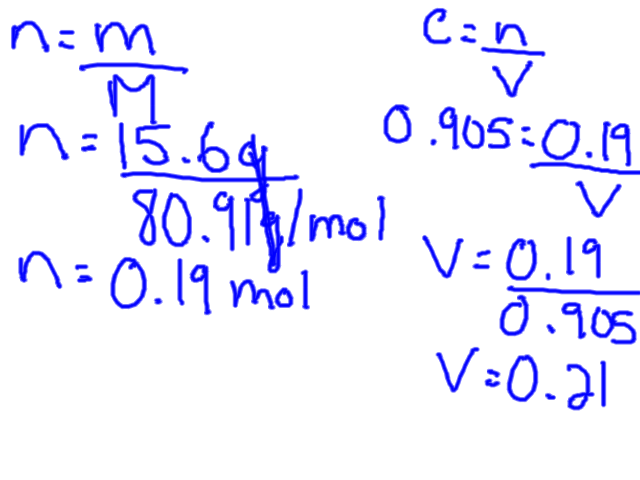
To calculate molarity you need the solute in moles, but usually you will have a certain weight of solute which means you first need to convert the grams into moles. This can be done by finding the molar mass of the solute from the periodic table.
The molar concentration formula can be rearranged to solve for both volume and moles.
Volume is influenced by changes in temperature or pressure. For instance, the volume would increase with increasing temperature. This means that there will be some question of accuracy where there are changes in temperature.
If temperature decreases enough then the liquid may contract causing the molarity to increase because the same number of moles remains but there would be less solution.
Conversely, if the temperature increases enough then the liquid may expand causing the molarity to decrease because the same number of moles remains but there would be more solution present.
Molarity can be used to calculate the concentration of a substance that has been diluted.
Molarity can be used when exact precision is not required. It is however influenced by changes in temperature because it is a volumetric measurement, so in some cases it may not be appropriate to use.
Molarity and molality can be the same in some cases. For instance 1 liter of water weighs 1 kg.
What is molality?
Molality can be defined as the number of moles of substance (known as the solute) that is found in a certain mass of solvent given in kg, that it is dissolved in.
The formula for calculating molality is :
m = mole solute / kg solvent
Molality is also referred to as molal concentration.
An example of making a molal concentration would be to weight out a certain amount of sugar for instance. This then needs to be converted into the number of moles using the molecular mass of the sugar. A beaker of water is then weighed and water added to the beaker until it weighs 1 kg.
The sugar is then added to the beaker of water and dissolved.
The advantage of molality over molarity is that it is unaffected by changes in temperature and pressure since it is calculated based on mass and not volume. The mass of the solvent is not affected by temperature in the way that the volume of a substance is, therefore molality is a more accurate measure of concentration than molarity.
In the case of water the molarity and molality may be the same since 1 liter of water weighs 1 kg, however this may not be the case with all liquids.
This means that molality needs to be used when colligative properties are concerned.
Molality is more accurate and provides a greater precision of concentration but takes longer to prepare as the solute has to be added to the weight of a solvent. If the solvent is liquid then this has to be weighed.
This can be done using a gravimetric system and an analytical balance to weigh the solvent.
What is the difference between molarity and molality?
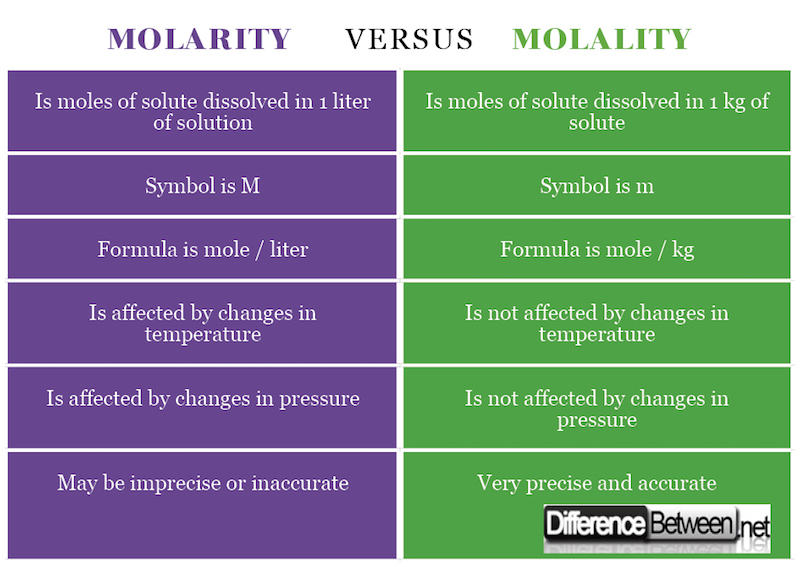
1) Molarity is concentration of a substance that is calculated as the number of moles of solute that is dissolved in 1 liter of solution while molality is concentration of a substance that is calculated as the number of moles of solute found in 1 kg of solvent.
2) The symbol for molarity is M, while that for molality is m (sometimes written as –m or m to distinguish it from mass).
3) The formula for molarity is moles / liter while the formula for molality is moles / kg.
4) Molarity is affected by changes in temperature while molality is unaffected by changes in temperature.
5) Molarity is affected by changes in pressure while molality is unaffected by changes in pressure.
6) Molarity may result in an imprecise and inaccurate concentration, while molality results in an accurate precise measurement of concentration.
Table comparing molarity and molality
Summary of Molarity and Molality
- Both molarity and molality can both be used to measure concentration.
- Molarity is defined as the number of moles of a solute that are dissolved in 1 liter of a solution.
- Molality is defined as the number of moles of a solute that are dissolved in 1 kg of a solvent.
- Molality is a more precise and accurate means of making a certain concentration because it is unaffected by temperature and pressure changes.
- Molarity involves a liquid which means that the concentration can change. This is because the volume being a liquid can change with changes in temperature and pressure.
- Difference Between Rumination and Regurgitation - June 13, 2024
- Difference Between Pyelectasis and Hydronephrosis - June 4, 2024
- Difference Between Cellulitis and Erysipelas - June 1, 2024
Search DifferenceBetween.net :
7 Comments
Leave a Response
References :
[0]Encyclopedia Britannica. “molality”. Science. Encyclopedia Britannica, 2017, https://www.britannica.com/science/molality
[1]Robinson, William R. et al. Chemistry. Houston: Rice University, 2017. Print.
[2]ThoughtCo. “molarity-definition-in-chemistry”. ThoughtCo. ThoughtCo, 2017, https://www.thoughtco.com/molarity-definition-in-chemistry-606376.
[3]Image Credit: https://www.flickr.com/photos/7630430@N04/2365549016/in/photolist-4B34iS-HESBT9-9mkpSk-FG6uu-bAqBcZ-cefFvN-dPbBeD-dq7fE5-SC62VT-5zKaAH-bRc1FH-5GEXiH-6uYThd-bUSc4x-edey5i-bChiGh-x4qjn8-5GEYmT-6ihvmL-bChtN7-bChtJJ-bChtMq-bRaWKx-ff3LkU-w6Q8K7-bRcbAT-bUSfQX-dPbBfk-x1bPLj-bChiHm-bChiJo-bChiNo-bRc1CX-bChtKu-bRaWPV-o4YQd4-bb4vxF-6hjyCB-NXhKkv-tCjWa-TijEex-xDSBdm-9tCi8S-8ACxLg-qKYiVR-c7YX37-tka9pq-x4phke-tonPmG-PW6G3b
[4]Image Credit: https://www.flickr.com/photos/83960974@N05/7689514732/

Very good website i love it
vry gud explanation
These explanations have helped me a lot….
Thanks this was very useful and helpful:)
This is so very helpful
Not informative..
Hi,
Thank you for all the information.
There is a small typo in the the second column of the first row of the table molarity vs molality, it states:
“Is moles of solute dissolved in 1 kg of solute”
The author meant:
“Is moles of solute dissolved in 1 kg of solvent”
Keep up the great work.