Difference Between Acetone and Water
What is Acetone?
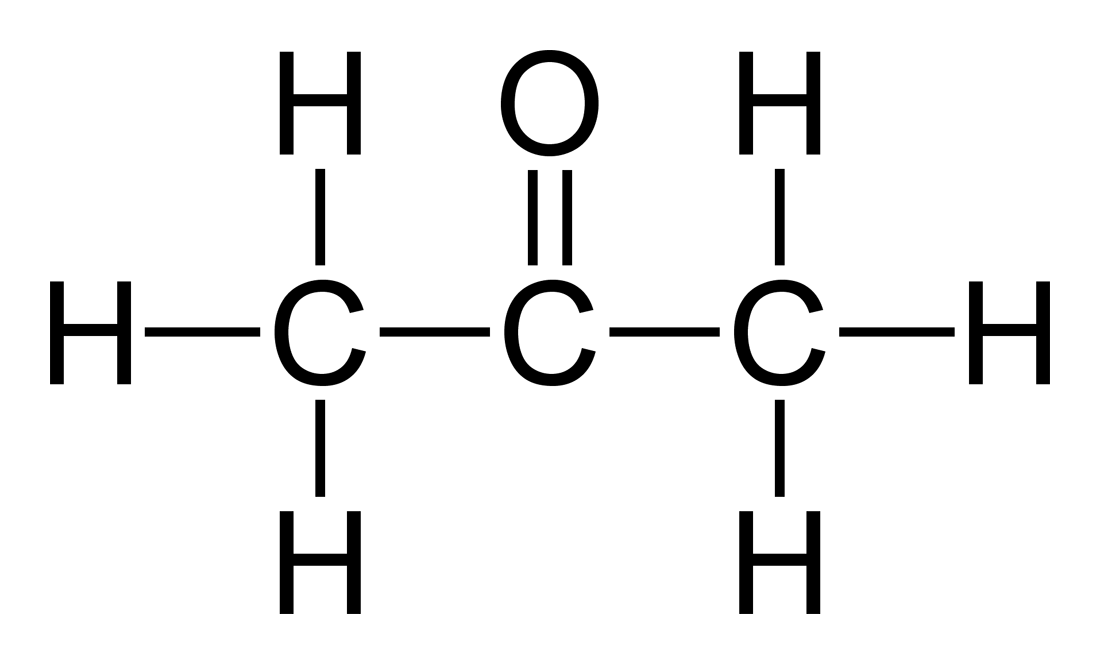
Acetone is the simplest ketone, also known as dimethyl ketone or propanone. Its chemical formula is C3H6O. The acetone is a colorless, highly flammable liquid with a specific odor.
Acetone is produced by the moderate oxidation of 2-propanol in the presence of a copper catalyst.
It can be dissolved in water, ethanol, ester, and other solvents.
The most common use of acetone is in domestic nail polish cleaner. It is also used in the production of plastics, film strips, acetate silk, synthetic rubber, smokeless gunpowder, some medicines, etc. As a good solvent for many polar and non-polar organic compounds, it is used to clean contaminated surfaces, as a solvent for paints, varnishes, various organic synthetic reactions, etc. Many of the plastics dissolve or swell in acetone.
Acetone occurs in animals, plants, vehicle exhaust, forest fires, volcanic gases, etc.
Acetone occurs in the human body in small quantities. After prolonged starvation or diet the stock of carbohydrates in the body is depleted and the fat is decomposed, which leads to the production of acetone. As a result, the so-called “acetone breath” occurs. More severe cases result in ketoacidosis, which is also one of the symptoms of diabetes.
Inhaled acetone vapor causes dizziness and intoxication. Acetone dissolves fats very well, so it causes dry and cracked skin.
The molecular weight of acetone is 58.08 g/mol. Its vapors are two times heavier than air. The density of acetone related to water is 0.8 (water = 1). It is stable under the recommended storage conditions.
Acetone’s boiling point is 56°C, and its melting point is -95°C. Auto-ignition occurs at 465°C.
What is Water?
Water is an inorganic compound with a chemical formula H2O. At room temperature, it is a transparent, odorless, and colorless liquid.
In general, the water is derived from the available natural sources. However, alternative water extraction technologies are developed for dry areas where no water sources are available. For example in Morocco is built the world’s largest system for extracting water from the air by fog condensation, covering over 600 square meters.
In Europe about 42% of utilized water is used in agriculture, 32% for industrial needs, 18% for energy production, and 8% for domestic purposes. In the US about 70% of the utilized water is used in agriculture, around 22% for industrial needs and power production, and about 8% – for domestic purposes.
Water covers about 71% of the surface of the Earth, mostly in oceans and seas. About 1.7% of the water occurs as groundwater and 1.7% of it is frozen in glaciers and the ice caps.
In the human body, there are about 70 – 75% of water. The amount may slightly vary and the following general dependencies are observed:
- Babies and children have a higher percentage of water than adults;
- Women have a smaller percentage of water than men;
- Overweight people have a smaller percentage of water than those of normal weight.
All life processes in the human body are connected with water. Water dissolves nutrients, removes waste products. It provides metabolism and is the environment in which all biochemical processes take place.
The water is one of the least toxic compounds. However, if it is being consumed in a high quantity without adequate electrolyte intake water intoxication can occur.
The molecular weight of the water is 18.02 g/mol. Its vapors are lighter than air.
Water’s boiling point is 100°C, and its melting point is 0°C. Water is a stable compound. It is not combustible and cannot be ignited.
Difference Between Acetone and Water
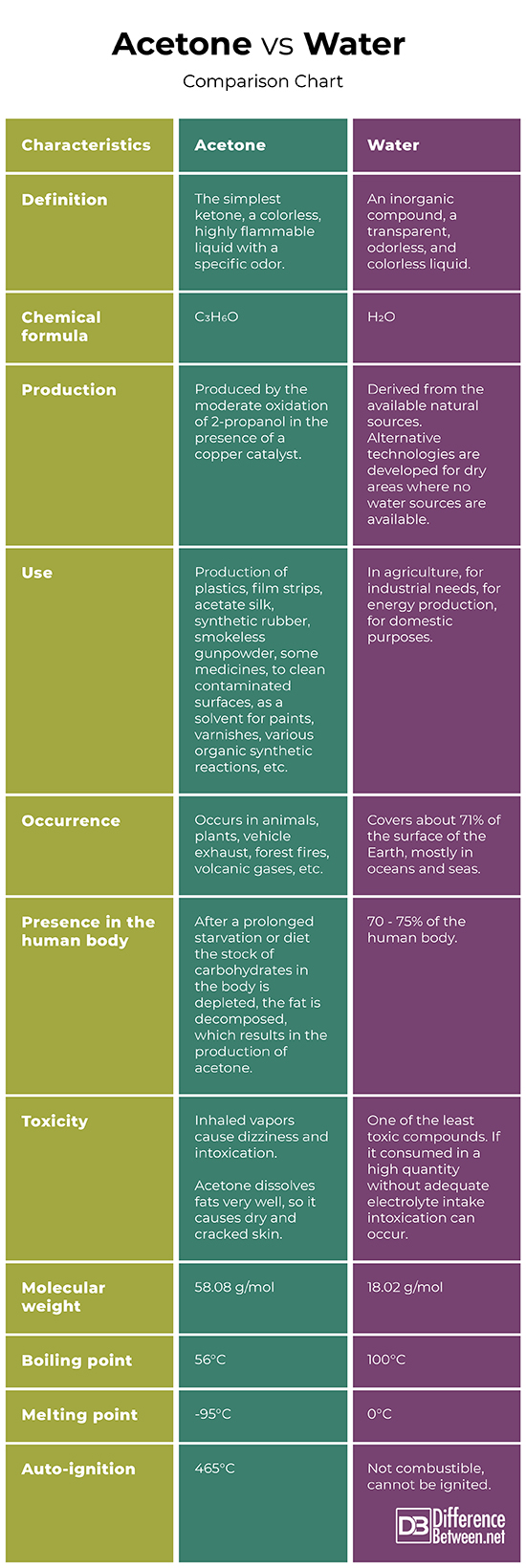
Definition
Acetone: Acetone is the simplest ketone, a colorless, highly flammable liquid with a specific odor.
Water: Water is an inorganic compound, a transparent, odorless, and colorless liquid.
Chemical formula
Acetone: The chemical formula of acetone is C3H6O.
Water: The chemical formula of water is H2O.
Production
Acetone: Acetone is produced by the moderate oxidation of 2-propanol in the presence of a copper catalyst.
Water: The water is derived from the available natural sources. Alternative water extraction technologies are developed for dry areas where no water sources are available.
Use
Acetone: Acetone is used in the production of plastics, film strips, acetate silk, synthetic rubber, smokeless gunpowder, some medicines, to clean contaminated surfaces, as a solvent for paints, varnishes, various organic synthetic reactions, etc.
Water: Water is used in agriculture, for industrial needs, for energy production, and for domestic purposes.
Occurrence
Acetone: Acetone occurs in animals, plants, vehicle exhaust, forest fires, volcanic gases, etc.
Water: Water covers about 71% of the surface of the Earth, mostly in oceans and seas. About 1.7% of the water occurs as groundwater and 1.7% of it is frozen in glaciers and the ice caps.
Presence in the human body
Acetone: After a prolonged starvation or diet the stock of carbohydrates in the body is depleted, the fat is decomposed, which results in the production of acetone.
Water: In the human body, there are about 70 – 75% of water.
Toxicity
Acetone: Inhaled acetone vapor causes dizziness and intoxication. Acetone dissolves fats very well, so it causes dry and cracked skin.
Water: The water is one of the least toxic compounds. However, if it is being consumed in a high quantity without adequate electrolyte intake water intoxication can occur.
Molecular weight
Acetone: The molecular weight of acetone is 58.08 g/mol.
Water: The molecular weight of water is 18.02 g/mol.
Boiling and melting point
Acetone: Acetone’s boiling point is 56°C, and its melting point is -95°C.
Water: Water’s boiling point is 100°C, and its melting point is 0°C.
Auto-ignition
Acetone: Acetone’s auto-ignition occurs at 465°C.
Water: Water is not combustible and cannot be ignited.
Acetone Vs. Water: Comparison Chart
Summary of Acetone vs. Water:
- Acetone is the simplest ketone, a colorless, highly flammable liquid with a specific odor.
- Water is an inorganic compound, a transparent, odorless, and colorless liquid.
- The chemical formula of acetone is C3H6O and the chemical formula of water is H2O.
- Acetone is produced by the moderate oxidation of 2-propanol in the presence of a copper catalyst. Water is derived from the available natural sources.
- Acetone is used in the production of plastics, film strips, acetate silk, synthetic rubber, smokeless gunpowder, some medicines, to clean contaminated surfaces, as a solvent for paints, varnishes, various organic synthetic reactions, etc. Water is used in agriculture, for industrial needs, for energy production, and for domestic purposes.
- Acetone occurs in animals, plants, vehicle exhaust, forest fires, volcanic gases, etc. Water covers about 71% of the surface of the Earth.
- After a prolonged starvation or a diet the stock of carbohydrates in the body is depleted, the fat is decomposed, which results in the production of acetone. In the human body, there are about 70 – 75% of water.
- Acetone is toxic. Water is one of the least toxic compounds.
- The molecular weight of acetone is 58.08 g/mol and the molecular weight of water is 18.02 g/mol.
- Acetone’s boiling point is 56°C, and its melting point is -95°C. Water’s boiling point is 100°C, and its melting point is 0°C.
- Acetone’s auto-ignition occurs at 465°C. Water is not combustible and cannot be ignited.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
1 Comment
Leave a Response
References :
[0]Image credit: https://commons.wikimedia.org/wiki/File:H2O_water_vapor_icon.png
[1]Image credit: https://commons.wikimedia.org/wiki/File:Acetone-2D-flat.png
[2]De, A. A Text Book of Inorganic Chemistry. New Delhi: New Age Publishers. 2003. Print.
[3]Hoffman, R. Organic Chemistry (2nd Edition). Hoboken: John Wiley & Sons, Inc. 2004. Print.
[4]Kirkova, E. General Chemistry. Sofia: Kliment Ohridski. 2002. Print.

Wake Him Up