Difference between Physical and Chemical Properties
What is physical properties?
Physical properties are those that can be observed and measured without changing the actual composition of the matter. The chemical and molecular composition remains the same regardless of the measurement method that is used.
Any property that can be detected and measured without performing a chemical reaction is thus a physical property.
Physical changes can occur, e.g. change of states, but this only changes the physical shape not the chemical structure or molecular composition of the substance. For instance, when water freezes, the chemical nature of the water does not change, so freezing point is another physical property.
States of matter is also a physical property since all substances can exist in a solid, liquid or gas phase depending on energy loss or gain.
The same element is present after the change and throughout the process. Physical changes are related to physical properties.
Physical properties can be extensive or intensive:
- Extensive –depends on the amount of matter being measured, for instance, mass, volume, and length.
Extensive properties are external, that is the substance can’t be identified using these and the value changes depending on the amount of the substance present. For example you can measure 10g of oil or 10g of water but this doesn’t enable you to identify a substance as being oil or water.
- Intensive – do not depend on the amount of matter being measured, for instance: color, density, viscosity, buoyancy, melting point, freezing point.
Intensive properties are always the same and can be used to identify what a substance is. E.g. density of liquid water is 1g/ml, boiling point is 100oC and freezing point is 0oC.
Using multiple intensive properties together allows one to identify a substance. Substances can also be classified and grouped based on their physical properties.
Examples of physical properties include:
- Temperature
- Malleability
- Appearance
- Texture
- Color
- Odor
- Shape
- Solubility
- Electric charge
- Molecular weight
- Boiling point
- Melting point
- Freezing point
- Volume
- Mass
- Length
- Density
- Solubility
- Polarity
- Viscosity
- Pressure
- Electric charge
- Hardness
What is chemical properties?
A chemical property by definition means that measuring the property leads to a change in the actual chemical structure of the substance. Chemical properties become apparent when the substance undergoes a chemical change or reaction.
Chemical properties describe the ability of a substance to combine with other substances, or change into a different product. It is a way to describe what a substance could react with or end up changing into. When a chemical reaction occurs, matter changes to an entirely different type of matter.
For instance sodium can react with water vapor in the air and violently explode; iron and oxygen combine to form rust so iron has the chemical ability to form rust; gasoline has the ability to burn (it is flammable).
A chemical property is any quality that can be established only when a change is made in the chemical identity of the substance. Simply touching or observing a substance will not demonstrate its chemical properties. The structure of the matter or substance has to be changed in order to see the chemical property.
Chemical properties are useful to know since this helps in the identification of unknown substances or when trying to separate or purify substances, and can enable scientists to classify substances such as compounds.
Knowing these properties, scientists can come up with applications where various substances can be used.
Scientists are also able to predict how samples will react in a chemical reaction if they have prior knowledge of the substances’ chemical properties.
Some examples of chemical properties include the following:
- Toxicity
- Chemical stability (if a compound will react with water or air)
- Heat of combustion
- Flammability (whether compound will burn when exposed to flame)
- Reactivity (ability to react with other chemicals)
- Enthalpy of formation
- Oxidation states (gaining oxygen, losing hydrogen, or losing electrons, and resulting in the oxidation number of a substance being changed. An example of this would be rust).
- Types of chemical bonds that will form (whether covalent, noncovalent or hydrogen)
- Buoyancy
- Viscosity
- Compressibility
- Radioactivity (emission of radiation from an atom)
- Half-life
What is the difference between physical and chemical properties?
- Physical properties are those properties that can be observed or measured without
causing or resulting in a change in the matter, while chemical properties are only observed after a change in the matter has occurred.
- Physical properties can change states without changing the molecular structure, but this is not the case for chemical properties.
- With chemical properties the chemical identity of the substance is changed, this is not the case with physical properties.
- With chemical properties the structure of the material changes, while the structure does not change in the case of physical properties.
- A chemical reaction occurs before a chemical property becomes evident, while no chemical reaction is needed for a physical property to become visible.
- Chemical properties, unlike physical properties, can be used to predict how substances will react.
Table comparing physical and chemical properties
| Physical property | Chemical property |
| Observed without bringing about a change | Only observed after bringing about a change |
| Can change physical state but not molecules | Always changes molecules |
| Chemical identity remains the same | Chemical identity changes |
| Structure of material does not change | Structure of material changes |
| No chemical reaction is needed to show the property | Chemical reaction is needed to show property |
| Can’t be used to predict how substances will react | Can be used to predict how substances will react |
Summary:
- Physical properties can be observed without having to undergo any change in the matter.
- Physical properties can vary depending on the amount of matter, for instance, length, volume and mass. These are known as extensive physical properties.
- Intensive physical properties don’t depend on the amount of matter, e.g. texture.
- Physical properties can change states but still retain the same chemical structure, e.g. water freezing or boiling.
- Chemical properties can only be observed with a change, such as a chemical reaction.
- Matter is classified both based on their physical and chemical properties.
- Difference Between Rumination and Regurgitation - June 13, 2024
- Difference Between Pyelectasis and Hydronephrosis - June 4, 2024
- Difference Between Cellulitis and Erysipelas - June 1, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Ehret, William Frederick, Howard Elmer Wahlert, Carel Willem Van der Merwe, Walter A.b. Schneider, and Leslie Erskine Spock. Physical Science. New York: Macmillan, 1942. Print.
[1]Ma, Samantha. “Physical and chemical properties of matter”. Chemistry. Libretexts, 2017, https://chem.libretexts.org/Core/Inorganic_Chemistry/Chemical_Reactions/Properties_of_Matter
[2]Ophardt, Charles E. “Physical Properties”. Virtual Chembook. Elmhurst College, 2003, http://chemistry.elmhurst.edu/vchembook/104Aphysprop.html.
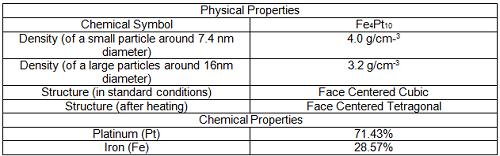
[3]"Image Credit: https://commons.wikimedia.org/wiki/File:FePt_Physical_Properties.png"
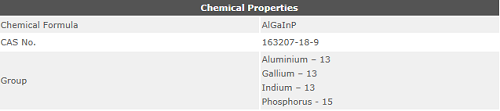
[4]"Image Credit: https://commons.wikimedia.org/wiki/File:Chemical_Properties.PNG"