Difference Between Benzyl and Phenyl
Functional group is a group of atoms with distinctive chemical properties. Those groups are responsible for the characteristic chemical properties of the molecules they build.
Benzyl and phenyl are functional groups, containing a benzene ring.
What is Benzyl?
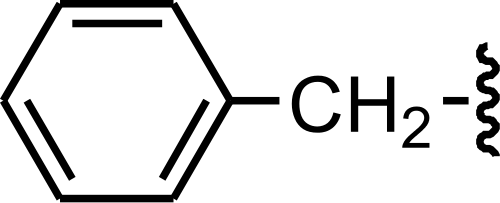
Benzyl is a functional group, consisting of a benzene ring, attached to a CH2 group. Its chemical formula is C6H5CH2–. Benzyl is a monovalent radical derived from toluene.
The abbreviation “Bn” is used to represent the benzyl group. For example, the benzyl alcohol can be marked as BnOH.
The position of the first C atom bonded to an aromatic ring is described as benzylic. The benzylic positions are characterized by enhanced reactivity. It is due to the low dissociation energy of the benzyl C−H bonds. The energy, necessary for bond dissociation of the benzyl C-H bond is 90 kcal/mol, while for the methyl C−H bond it is 105 kcal/mol, and for the ethyl C−H bond it is 101 kcal/mol.
The aromatic ring has a stabilizing role for the benzyl radicals. However, the weak C−H bond impacts the stability of the benzyl radical. Because of the weaker C-H bond, benzylic substituents show enhanced reactivity in free radical halogenation, oxidation, hydrogenolysis, etc.
Chemical compounds, containing a benzyl group are benzyl methyl, benzyl bromide, benzyl chloroformate, benzyl amine, etc.
Benzyl is used in organic synthesis as a protecting group for carboxylic acids and alcohols.
What is Phenyl?
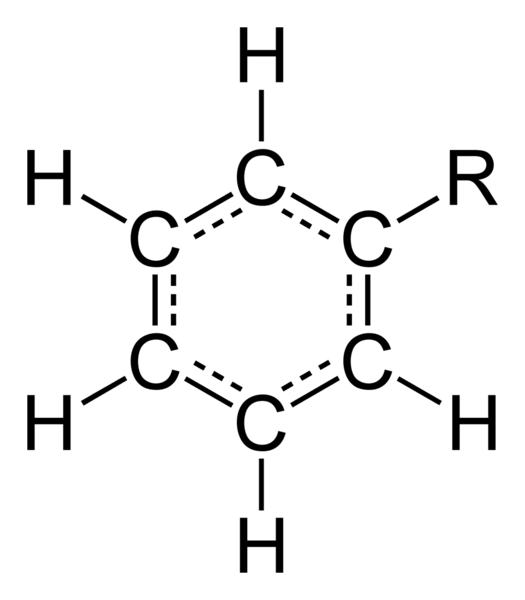
Phenyl is a cyclic functional group with the formula C6H5-. It is a monovalent aryl radical, closely related to benzene and derived from it by removal of one H atom. The phenyl group has six C atoms bonded in a hexagonal ring. One of these atoms is bonded to a substituent, and the other five are bonded to hydrogen atoms.
The abbreviation “Ph” is used to represent the phenyl group. For example, the benzene can be denoted as PhH.
The dissociation energy of the phenyl C−H bonds is higher than this of other C-H bonds. The energy necessary for bond dissociation of the phenyl C-H bond is 113 kcal/mol, while for the methyl C−H bond it is 105 kcal/mol, and for the ethyl C−H bond it is 101 kcal/mol.
The properties of the aromatic molecular orbitals additionally increase the stability of the phenyl group. Phenyl substances are hydrophobic and tend to resist reduction and oxidation.
Chemical compounds, containing phenyl group are triphenylmethane, chlorobenzene, phenol, etc.
Compounds containing phenyl groups are used in the medicine. For example, Atorvastatin is used to lower cholesterol, Fexofenadine is used to treat allergies.
Difference Between Benzyl and Phenyl
-
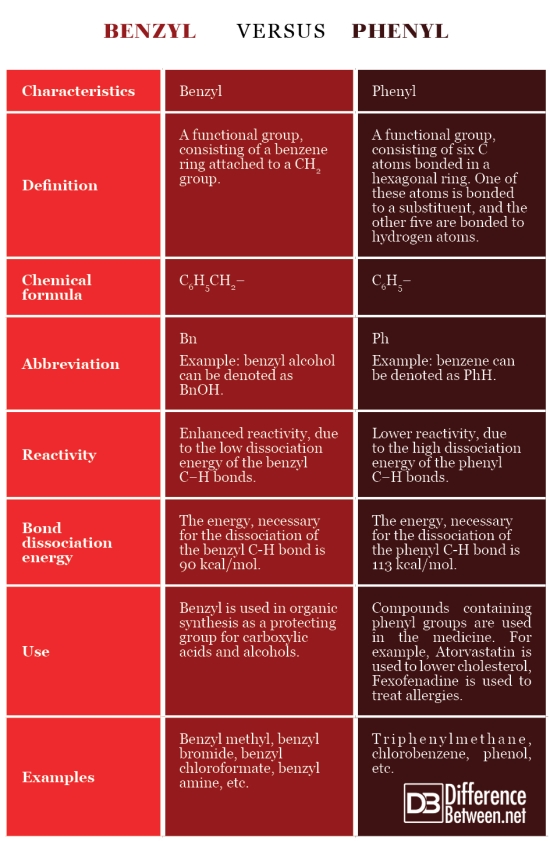
Definition
Benzyl: Benzyl is a functional group, consisting of a benzene ring attached to a CH2 group.
Phenyl: Phenyl is a functional group consisting of six C atoms bonded in a hexagonal ring. One of these atoms is bonded to a substituent, and the other five are bonded to hydrogen atoms.
-
Chemical formula
Benzyl: The chemical formula of benzyl is C6H5CH2–.
Phenyl: The chemical formula of phenyl is C6H5–.
-
Abbreviation
Benzyl: The abbreviation “Bn” is used to represent the benzyl group. For example, the benzyl alcohol can be marked as BnOH.
Phenyl: The abbreviation “Ph” is used to represent the phenyl group. For example, the benzene can be denoted as PhH.
-
Reactivity
Benzyl: The benzylic positions are characterized by enhanced reactivity, due to the low dissociation energy of the benzyl C−H bonds. Benzylic substituents show enhanced reactivity in free radical halogenation, oxidation, hydrogenolysis, etc.
Phenyl: The phenylic positions are characterized by lower reactivity, due to the high dissociation energy of the phenyl C−H bonds. Phenyl substances are hydrophobic and tend to resist reduction and oxidation.
-
Bond dissociation energy
Benzyl: The energy, necessary for the dissociation of the benzyl C-H bond is 90 kcal/mol.
Phenyl: The energy, necessary for the dissociation of the phenyl C-H bond is 113 kcal/mol.
-
Use
Benzyl: Benzyl is used in organic synthesis as a protecting group for carboxylic acids and alcohols.
Phenyl: Compounds containing phenyl groups are used in the medicine. For example, Atorvastatin is used to lower cholesterol, Fexofenadine is used to treat allergies.
-
Examples
Benzyl: Chemical compounds, containing benzyl group are benzyl methyl, benzyl bromide, benzyl chloroformate, benzyl amine, etc.
Phenyl: Chemical compounds, containing phenyl group are triphenylmethane, chlorobenzene, phenol, etc.
Benzyl Vs. Phenyl Comparison Chart
Summary of Benzyl Vs. Phenyl
- Functional group is a group of atoms with distinctive chemical properties.
- Benzyl is a functional group, consisting of a benzene ring attached to a CH2 group.
- Phenyl is a functional group consisting of six C atoms bonded in a hexagonal ring. One of these atoms is bonded to a substituent, and the other five are bonded to hydrogen atoms.
- The chemical formula of benzyl is C6H5CH2–, while the chemical formula of phenyl is C6H5–.
- The abbreviation “Bn” is used to represent the benzyl group, while the abbreviation “Ph” is used to represent the phenyl group.
- The benzylic positions are characterized by enhanced reactivity, due to the low dissociation energy of the benzyl C−H bonds. The phenylic positions are characterized by lower reactivity, due to the high dissociation energy of the phenyl C−H bonds.
- Benzyl substituents show enhanced reactivity in free radical halogenation, oxidation, hydrogenolysis, etc. Phenyl substances are hydrophobic and tend to resist reduction and oxidation.
- The energy, necessary for the dissociation of the benzyl C-H bond is 90 kcal/mol. The energy, necessary for the dissociation of the phenyl C-H bond is 113 kcal/mol.
- Chemical compounds, containing benzyl group are benzyl methyl, benzyl bromide, benzyl chloroformate, benzyl amine, etc. Chemical compounds, containing phenyl group are triphenylmethane, chlorobenzene, phenol, etc.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
Leave a Response
References :
[0]Hoffman, R. Organic Chemistry (2nd Edition). Hoboken: John Wiley & Sons, Inc. 2004. Print.
[1]Kirkova, E. General Chemistry. Sofia: Kliment Ohridski. 2002. Print.
[2]Petrov, G. Organic Chemistry. Sofia: Kliment Ohridski. 2006. Print.
[3]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/f/f4/Benzyl_group.svg/500px-Benzyl_group.svg.png
[4]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/e/ef/Phenyl-group-2D-flat.png/525px-Phenyl-group-2D-flat.png