The Differences Between Urethane and Polyurethane
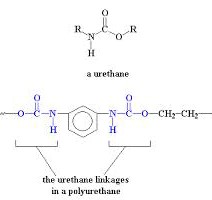
Each day we use several products made of organic compounds that influence our lifestyles. Urethane and polyurethane are the first among them. Many think the only difference between the two is that polyurethane is produced by linking urethane organic units. As you see in the picture below, far more dissimilarities exist even though the names are sometimes substituted for one another.
Urethane
Urethane is a synthetic crystalline compound used in the manufacture of pesticides, fungicides, cosmetics, and pharmaceuticals. Previously, it was considered to be an effective veterinary anesthetic. “Ethyl carbamate” and “ethyl urethane” are synonyms for urethane. Though polyurethane products may generally be known as “urethanes,” polyurethane is not at all the same as ethyl carbamate (called urethane). Polyurethanes do not contain ethyl carbamate and are not produced from it. Urethane is only a chemical group, while polyurethane is a material consisting of several urethane groups. These units of urethane have a definite number of atoms of oxygen, nitrogen, carbon, and hydrogen arranged in a particular pattern.
Urethane is obtained through the chemical reaction of polyol and isocyanate. It can be molded into any desired form or type by using the method of “open casting” or “compression.” Even though each product varies according to its chemical structure and regardless of whether it is plastic, varnish, or adhesive, all of them contain urethanes. A product made of polyurethane only means that it contains multiple urethanes. However, urethane is not a constituent of paints. Also, urethane cannot be considered as a proper description for resins used in them.
Urethane is a tough and hard polymer. The plastic made of urethane is durable and stable and has better compression properties. It is resistant to scratches, ozone, oxygen, etc., and maintains its shape and size for long periods. Urethane is odorless and colorless but has a bitter taste. As it is highly toxic, its application in pharmaceuticals has already diminished. It has been discovered that nausea may occur in people who take medicines having urethane content.
Polyurethane
Polyurethane is a polymer that contains urethane groups in its chains. It is capable of polymerizing with several carbamate groups to yield different amounts of chemical and moisture levels. Polyurethane is the common term attributed to the class of polymers produced through the intricate process of synthesis between isocyanate and polyols. The quality of the polymer relies much on the chemical properties as well as the quantity of its components, along with its processing conditions. Polyurethane is vulnerable to biodegradation due to microorganisms.
Polyurethane was first introduced in the year 1937 by Professor Dr. Otto Bayer through his invention of the “diisocyanate polyaddition process.” The polymer he developed had more advantages over the plastics obtained from polymerizing olefins. During the Second World War, the development of the polymer was limited to flexible foams and fibers. The outcomes were the mustard-gas-resistant garments, high-gloss aircraft finishing, and corrosion-resistant coatings for wood, metal, and masonry. And in 1954 when large-scale production of flexible polyurethane foam began, it paved the way for a renewed enthusiasm for inventing multifarious applications of polyurethanes. Later years witnessed the development of thousands of advanced polyurethane products and urethane variants, namely linear, castable, millable, thermoplastic, cellular, sprayable, poromeric, and spandex fiber polyurethanes.
Polyurethane is now widely used in the manufacture of medicines, automobiles, industrial products like floor and wall coatings, home insulation, solid plastics, and print rollers, etc. It is resistant to impact and abrasion and is a perfect substitute for plastic, rubber, and steel. The expanded polyurethane is spongy and deformable and is suitable for making pillows, mattresses, and car seats. It is not easily susceptible to color-fading, fungus, heat, oxidation, solvents, oil, or acid.
- Difference Between Flash Suppressor And Muzzle Brake - October 10, 2015
- The Differences Between Urethane and Polyurethane - October 9, 2015
- Difference Between Pelican And Stork - October 5, 2015
Search DifferenceBetween.net :
Leave a Response
References :
[0]National Center for Biotechnology Information. PubChem Compound Database; CID=5641, https://pubchem.ncbi.nlm.nih.gov/compound/5641 (accessed Sept. 15, 2015)
[1]http://pubchem.ncbi.nlm.nih.gov/compound/urethane#section=Computed- Properties
[2]Marois Y, Guidoin R. Biocompatibility of Polyurethanes. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000- . Available from: http://www.ncbi.nlm.nih.gov/books/NBK6422/
[3]http://oehs.vcu.edu/chemical/biosafe/urethane.pdf
[4]http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.473.7767
[5]http://www.housepaintingguide.org/what-is-the-difference-between-7. polyurethanes-and-urethanes/
[6]http://www.unomaha.edu/tiskochem/Chem4310/.../Polyurethane-McGill.ppt
[7]http://itech.dickinson.edu/chemistry/?p=920
[8]http://www.ncbi.nlm.nih.gov/books/NBK6422/
[9]http://www.precisionurethane.com/urethane-advantage.html
[10]http://uk.misumi-ec.com/pdf/fa/p2_0359.pdf
[11]http://www.ncbi.nlm.nih.gov/books/NBK6422/