Difference Between Polyurethane and Polycrylic
What is Polyurethane?
Polyurethane is a polymer (polyester). It is composed of organic units (isoamines and alcohols) joined by urethane links.
Polyurethane polymers are produced by a reaction between polyol (R−(OH)n) and di- or tri- poly-isocyanate (R−(N=C=O)n). The production is activated by UV light or by a catalyst.
Polyurethanes were made for the first time in Germany in 1937.
Polyurethanes are widely used in rigid foam insulation panels, hard-plastic parts, microcellular foam seals and gaskets, tires, wheels, high performance adhesives, electrical potting compounds, water and oil-based surface coatings, sealants, high-resilience foam seating, textiles, adhesives, packaging, paper-making, etc.
The properties of the polyurethane depend on the types of polyols and isocyanates used for its production. The great variety of possible combinations between isocyanates and polyols, additives, and processing conditions, leads to a great variety in the properties, which makes polyurethanes so widely used for so many different purposes.
For example, flexible, long segments, contributed by the polyol, give an elastic and soft polymer. Rigid or tough polymers are obtained as a result of high amounts of crosslinking. Limited crosslinking and long chains give very stretchy polymers, significant crosslinking and short chains result in a hard polymer, and so on.
A piece of polyurethane can be regarded as one huge molecule. The typical polyurethanes do not melt and are thermosetting polymers. However, a number of thermoplastic polyurethanes also exist.
Most of the thermosetting polyurethanes have very high abrasion resistance, tensile strength, toughness, and high degradation resistance.
What is Polycrylic?
Polycrylic is a brand name of a water-based protective paint. It is produced using polyacrylates and polyurethane. It contains copolymers of methacrylic acid, acrylic acid, and their esters.
Polycrylic is very resistant to water and is widely used to protect different wood and non-wood household items from different solvents and from water. Polycrylic provides protection, capable to withstand very rough conditions.
Unlike many of the oil-based polyurethanes, Polycrylic does not retain any color on the surface and is completely clear.
Difference Between Polyurethane and Polycrylic
-
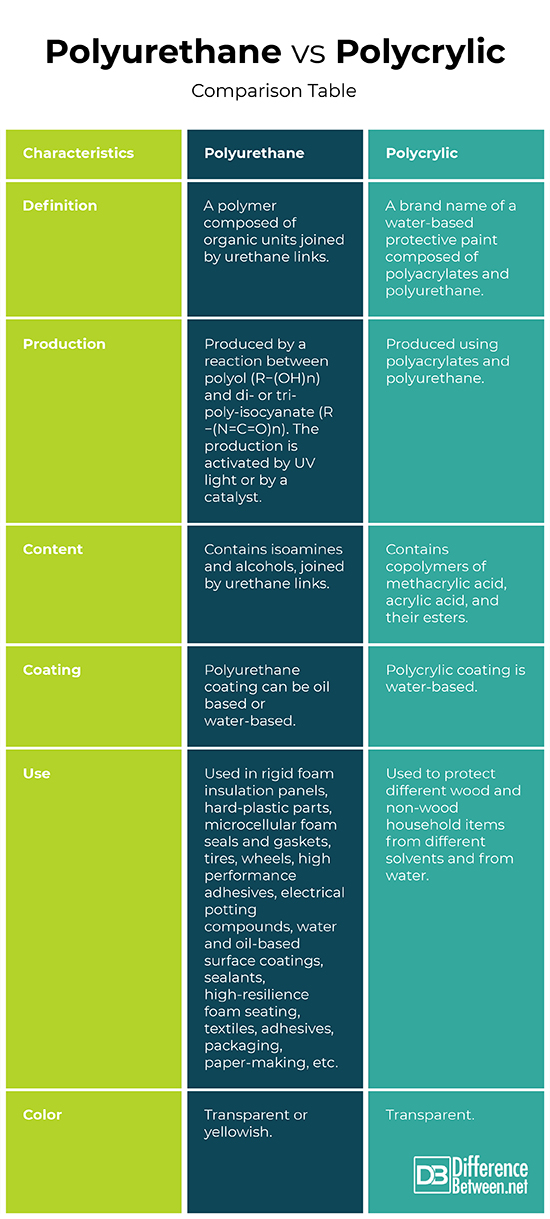
Definition
Polyurethane: Polyurethane is a polymer, composed of organic units joined by urethane links.
Polycrylic: Polycrylic is a brand name of a water-based protective paint, composed of polyacrylates and polyurethane.
-
Production
Polyurethane: Polyurethane is produced by a reaction between polyol (R−(OH)n) and di- or tri- poly-isocyanate (R−(N=C=O)n). The production is activated by UV light or by a catalyst.
Polycrylic: Polycrylic is produced using polyacrylates and polyurethane.
-
Content
Polyurethane: Polyurethane contains isoamines and alcohols, joined by urethane links.
Polycrylic: Polycrylic contains copolymers of methacrylic acid, acrylic acid, and their esters.
-
Coating
Polyurethane: Polyurethane coating can be oil based or water-based.
Polycrylic: Polycrylic coating is water-based.
-
Use
Polyurethane: Polyurethanes are widely used in rigid foam insulation panels, hard-plastic parts, microcellular foam seals and gaskets, tires, wheels, high performance adhesives, electrical potting compounds, water and oil-based surface coatings, sealants, high-resilience foam seating, textiles, adhesives, packaging, paper-making, etc.
Polycrylic: Polycrylic is used to protect different wood and non-wood household items from different solvents and from water.
-
Color
Polyurethane: The water-based polyurethane films are transparent, the oil-based are yellowish.
Polycrylic: Polycrylic films are transparent.
Polyurethane Vs. Polycrylic: Comparison Table
Summary of Polyurethane and Polycrylic
- Polyurethane is a polymer, composed of organic units joined by urethane links.
- Polycrylic is a brand name of a water-based protective paint, composed of polyacrylates and polyurethane.
- Polyurethane is produced by a reaction between polyol (R−(OH)n) and di- or tri- polyisocyanate (R−(N=C=O)n). The production is activated by UV light or by a catalyst. Polycrylic is produced using polyacrylates and polyurethane.
- Polyurethane contains isoamines and alcohols, joined by urethane links. Polycrylic contains copolymers of methacrylic acid, acrylic acid, and their esters.
- Polyurethane coating can be oil based, or water-based, while Polycrylic coating is only water-based.
- Polyurethanes are widely used in rigid foam insulation panels, hard-plastic parts, microcellular foam seals and gaskets, tires, wheels, high performance adhesives, electrical potting compounds, water and oil-based surface coatings, sealants, high-resilience foam seating, textiles, adhesives, packaging, paper-making, etc. Polycrylic is used to protect different wood and non-wood household items from different solvents and from water.
- The water-based polyurethane films are transparent, the oil-based are yellowish. Polycrylic films are always transparent.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://www.flickr.com/photos/49364825@N02/4526774736
[1]Image credit: https://www.flickr.com/photos/zieak/4156886133
[2]Polycrylic Safety Data Sheet. Upper Saddle River: MINWAX Company. 2018.
[3]Randall, D., S. Lee. The Polyurethanes Book. New York: Wiley. 2000. Print.
[4]Ulrich, H. Chemistry and Technology of Isocyanates. New York: John Wiley & Sons, Inc. 1996. Print.