Difference Between Covishield and Covaxin
The race to vaccinate the global population is on, with India leading the vaccine race against the deadly coronavirus pandemic. India has already proved its excellence with its massive inoculation drive and now with not just one but two potential vaccine candidates, it is about to create history.
We take a look at the two leading COVID-19 vaccine candidates – Bharat Biotech’s Covaxin and the SII’s Covishield.

What is Covishield?
Covishield, codenamed AZD1222 is a potential COVID-19 vaccine candidate developed by the University of Oxford and AstraZeneca, and locally manufactured by the world’s largest manufacturer of vaccines, Serum Institute of India (SII). After months of clinical trials, the Drugs Controller General of India (DGCI) has finally given a green light to the Covishield vaccine for restricted use in emergency situations.
The vaccine is believed to have a higher efficacy rate than other COVID-19 vaccine candidates, making it very effective against the Coronavirus. The vaccine is a giant leap towards the fight against the COVID-19 pandemic that has been wreaking havoc for months. Covishield is among the first vaccines to be approved for mass distribution.
On Jan. 16, 2021, India started rolling out the Covishield vaccines across the country in what was said to be a historic moment as the nation’s largest vaccination drive kicked off. The vaccination drive began with healthcare and frontline workers who were the first to receive the doses More than 220,000 people were reportedly given doses on the first day.
What is Covaxin?
Covaxin is the India’s first indigenously developed COVID-19 vaccine to have gained approval for the restricted emergency use in the fight against the pandemic. Covaxin is developed by a leading vaccine and bio-therapeutics manufacturer Bharat Biotech based out of Hyderabad, in collaboration with the Indian Council of Medical Research (ICMR) and Pune-based National Institute of Virology.
The effectiveness of the vaccine has been a question since it first started performing human trials on June 29, 2020, followed by controversies and mixed opinions regarding the emergency use of the vaccine despite it being an incompletely studied vaccine.
Despite the controversies, Bharat Biotech successfully finished the phase 3 clinical trials of the vaccine with impressive results, and on Dec. 7, 2020 applied for EUA to the DGCI for its vaccine. This is a great step forward, which pit Bharat Biotech’s vaccine against the SII’s vaccine and other COVID-19 vaccine candidates. According to a new preprint research paper, the Indian-made Covaxin is highly effective against the new variants, notably the UK variant.
Difference between Covishield and Covaxin
Vaccine Type
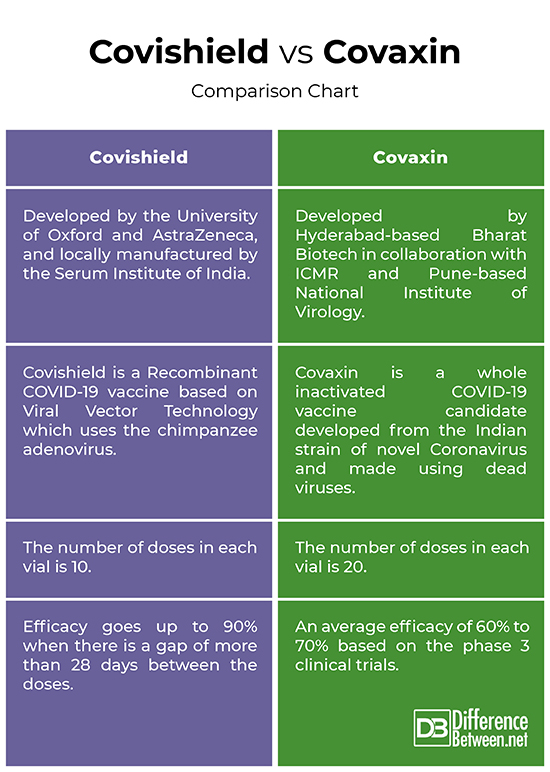
– Covishield, codenamed AZD1222, is a Recombinant COVID-19 vaccine based on Viral Vector Technology which uses the chimpanzee adenovirus that causes common cold in chimpanzees.
The vector vaccine contains the genetic codes for the SARS-CoV-2’s spike. A weakened strain of the adenovirus is injected into the body which is incapable of causing any infection. If successful, the body starts forming antibodies, blocking their entry into human cells and triggering an immune response.
The Bharat Biotech’s Covaxin, on the other hand, is a whole inactivated COVID-19 vaccine candidate developed from the Indian strain of novel Coronavirus isolated by National Institute of Virology.
The vaccine is made up of inactivated or dead viruses which have no potential impact to replicate or infect since it is already dead. The inactivated virus is then injected into the body into multiple doses to boost the immune response to make the antibodies fight against the virus.
Efficacy
– The Oxford-AstraZeneca Covishield vaccine which is locally manufactured by the Serum Institute of India and co-sponsored by the Indian Council of Medical Research for clinical trials, achieved an efficacy rate of 62.1% with over 23,000 candidates who received standard doses of two, and the participants who received a low dose followed by a full dose, the effectiveness of the vaccine hit 90%. However, reports suggest that if more than 28 days of gap is maintained between the two doses, the efficacy goes up significantly by 90-95%.
The inactivated Covaxin vaccine has showed an average efficacy of 60% to 70% based on the phase 3 clinical trials and the DGCI approved the vaccine as clinically safe and have a robust immune response with no potential side effects.
Although, no substantial data on the efficacy of the vaccine is produced so far, the vaccine is cleared for emergency authorization use. The vaccine is also highly effective against the emerging UK variant of the virus, according to a report published by the company.
Dosage
– While both the vaccines are administered as intramuscular injections, the number of doses in each vial is a little bit different. Covishield is administered as two separate doses of 0.5 ml each with the second dose scheduled 4 to 6 weeks after the first dose.
If the first dose is injected intramuscularly on Day 1, then the second dose should be injected on Day 29. It can be safely stored at 2 to 8 degrees Celsius, which makes it easy for domestic distribution and storage.
The number of doses in each vial of Covaxin is 20, which is double the amount of doses in Covishield at 10. The dosage is similar to that of Covishield’s – two doses 0.5 ml each scheduled 4 weeks apart after the first dose. The shelf-life is same 6 months and it can be stored at 2 to 8 degrees Celsius which is the same as a domestic refrigeration unit.
Covishield vs. Covaxin: Comparison Chart
Summary of Covishield vs. Covaxin
The reports suggest that when a larger number of doses need to be administered to the population, the Bharat Biotech’s Covaxin may be used. But in the beginning, the Serum Institute of India’s Covishield leads the COVID-19 vaccine race, with an efficacy of up to 95%.
The DGCI has given green lights to both Covaxin and Covishield for mass inoculation, making Indian the first country to be approved for not just one but two COVID-19 vaccines at one go. Covishield is a Recombinant COVID-19 vaccine based on Viral Vector Technology whereas Covaxin is a whole inactivated COVID-19 vaccine made using dead viruses.
- Difference Between JPEG and RAW - April 25, 2024
- Difference Between Serif and Sans Serif - April 22, 2024
- Difference Between HTML and Text - April 19, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Riegelman, Richard. COVID-19 Global Lessons Learned: Interactive Case Studies. Massachusetts, United States: Jones & Bartlett Learning, 2020. Print
[1]Romanowski, Victor. Current Issues in Molecular Virology: Viral Genetics and Biotechnological Applications. London, United Kingdom: Intech Open, 2013. Print
[2]Image credit: https://cultura.uol.com.br/upload/tvcultura/noticias/20210203214058_gettyimages-1230630497.jpg
[3]Image credit: https://commons.wikimedia.org/wiki/File:Oxford_AstraZeneca_vaccine_(Indian_version)_2021_D.jpg
