Difference Between Acetic Acid and Glacial Acetic Acid
What is Acetic Acid?
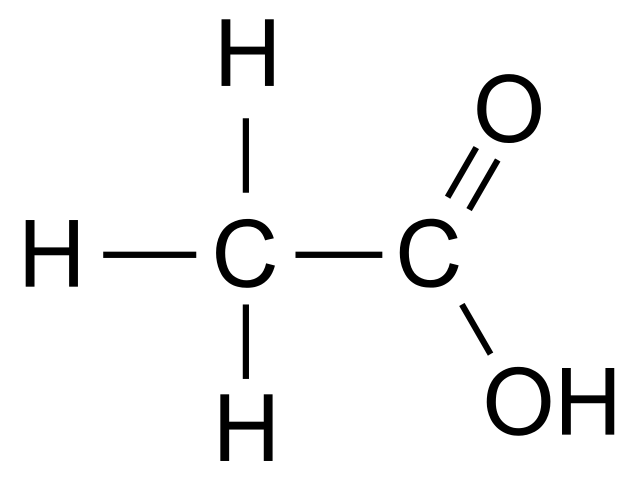
Acetic acid (CH3COOH) is one of the simplest carboxylic acids. Under the IUPAC system nomenclature, the name of acetic acid is ethanoic acid.
The acetic acid is synthesized in decomposition and acetic fermentation. In nature, it is found in plant and animal organisms, both in a free state and in the form of esters and other derivatives.
In the past, acetic acid has been produced by acetic fermentation of the ethyl alcohol contained in the wine. By the action of bacterial enzymes, the alcohol of the wine is oxidized by the oxygen of the air to acetic acid.
Industrial method for the production of acetic acid is the direct catalytic oxidation of acetaldehyde or butane. It may also be industrially produced by carbonylation of methanol, catalyzed by rhodium-iodine.
Acetic acid is a colorless liquid with a pungent smell and sour taste. It dissolves very well in water. Due to the greater polarity of the bond, the O-H carboxylic acids form stronger intermolecular hydrogen bonds than the alcohols, which determines the unrestricted solubility of acetic acid.
The chemical properties of acetic acid are determined by its carboxyl functional group and by the methyl moiety. The acid participates in chemical reactions with breakage of the bonds in the carboxyl group.
Acetic acid shows the typical chemical properties of the organic acids. In water solution the acetic acid is dissociated according to the equation:
CH3COOH → CH3COO¯ + H
The degree of electrolytic dissociation is significantly lower than that of strong inorganic acids, so acetic is a weak acid. It reacts with highly electropositive metals, basic oxides, basic hydroxides, and salts of weaker acids. The obtained salts are called acetates (ethanoates).
Reactions with oxides, hydroxides, and salts break the O-H bond in the carboxyl group.
The reaction of acetic acid with alcohols in the presence of strong acids is called esterification, it leads to the production of esters.
Acetic acid also participates in reactions affecting the methyl group – substitution reactions in the hydrocarbon moiety.
In the form of vinegar, acetic acid solutions (5 to 18%) are used in the food industry and in the households. Acetic acid is used for fixation of photographic films, to remove calcium deposits from cranes and boilers, to treat a jellyfish sting, etc. It is also used as a preservative for silage, as it inhibits the growth of bacteria and fungi.
What is Glacial Acetic Acid?
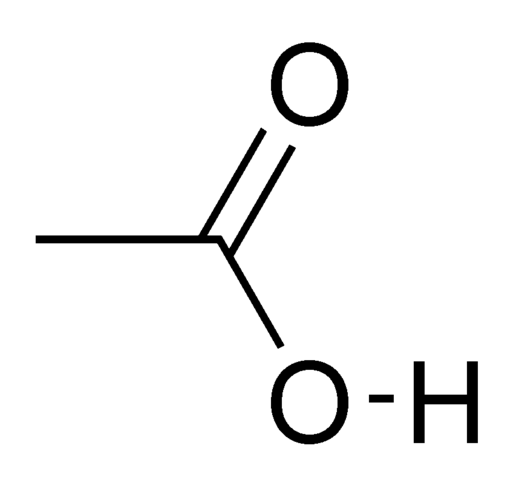
The pure, anhydrous acetic acid is a colorless, hygroscopic liquid. At temperatures below 16.7°C, it forms ice-like crystals. That’s why it is called glacial acetic acid.
The glacial acetic acid boils at high temperature (118 °C). Reason for this is the formation of stable hydrogen bonds between any two molecules of acetic acid in the form of a cyclic dimer. The point of flammability is 39°C. The density at 25 °C is 1.05 g/mL.
For centuries, chemists have thought that glacial acetic acid and acid in vinegar are two different substances.
The glacial acetic acid is corrosive and its vapors irritate the eyes and nose. In contact with eyes and skin, it can lead to injuries.
Upon contact of acetic acid with crystallized glacial acetic acid the pure acetic acid attaches to the crystal.
The glacial acetic acid is a great polar base solvent. It is often used in the production of:
- Terephthalic acid;
- Propylene terephthalate;
- Synthetic camphor;
- Aniline.
Difference Between Acetic Acid and Glacial Acetic Acid
-
Definition
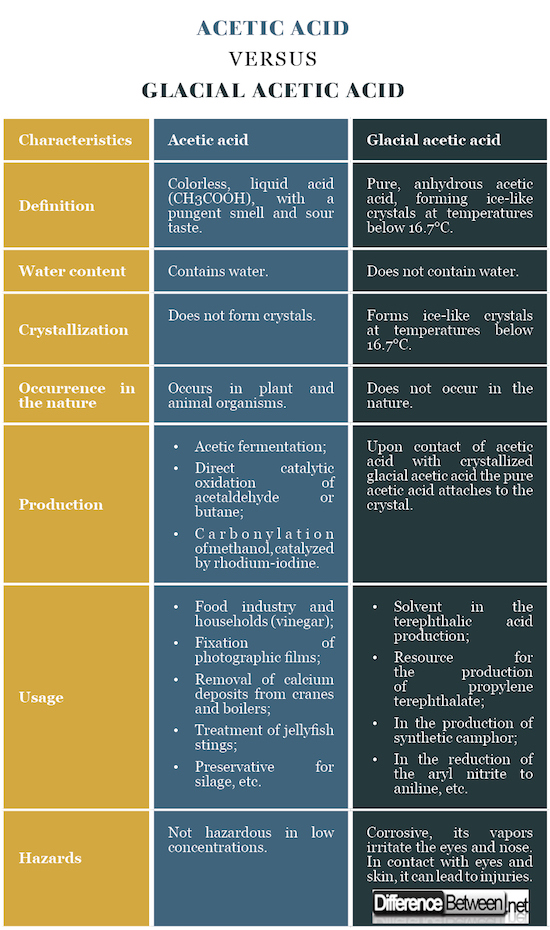
Acetic acid: The acetic acid is a colorless, liquid acid (CH3COOH), with a pungent smell and sour taste.
Glacial acetic acid: The pure, anhydrous acetic acid, forming ice-like crystals at temperatures below 16.7°C, is called glacial acetic acid.
-
Water content
Acetic acid: The acetic acid contains water.
Glacial acetic acid: The glacial acetic acid does not contain water.
-
Crystallization
Acetic acid: The acetic acid does not form crystals.
Glacial acetic acid: At temperatures below 16.7°C, the glacial acetic acid forms ice-like crystals.
-
Occurrence in the nature
Acetic acid: In the nature, the acetic acid is found in plant and animal organisms.
Glacial acetic acid: The pure, anhydrous acetic acid does not occur in the nature.
-
Production
Acetic acid: Acetic acid can be produced by acetic fermentation, by direct catalytic oxidation of acetaldehyde or butane, and by carbonylation of methanol, catalyzed by rhodium-iodine.
Glacial acetic acid: Upon contact of acetic acid with crystallized glacial acetic acid the pure acetic acid attaches to the crystal.
-
Usage
Acetic acid: The acetic acid is used in the food industry and in the households (vinegar); for fixation of photographic films; to remove calcium deposits from cranes and boilers; to treat a jellyfish sting, as a preservative for silage, etc.
Glacial acetic acid: The glacial acetic acid is used in the production of terephthalic acid, propylene terephthalate, synthetic camphor, aniline, etc.
-
Hazards
Acetic acid: The acetic acid is not hazardous in low concentrations.
Glacial acetic acid: The glacial acetic acid is corrosive and its vapors irritate the eyes and nose. In contact with eyes and skin, it can lead to injuries.
Difference Between Acetic Acid and Glacial Acetic Acid: Comparison Chart
Summary of Acetic Acid vs Glacial Acetic Acid
- The acetic acid is a colorless, liquid acid (CH3COOH), with a pungent smell and sour taste.
- The pure, anhydrous acetic acid, forming ice-like crystals at temperatures below 16.7°C, is called glacial acetic acid.
- The acetic acid contains water, while the glacial acetic acid does not.
- The acetic acid does not form crystals, while at temperatures below 16.7°C, the glacial acetic acid forms ice-like crystals.
- In the nature, the acetic acid is found in plant and animal organisms. The pure, anhydrous acetic acid does not occur in the nature.
- Acetic acid can be produced by acetic fermentation, by direct catalytic oxidation of acetaldehyde or butane, and by carbonylation of methanol, catalyzed by rhodium-iodine. Upon contact of acetic acid with crystallized glacial acetic acid the pure acetic acid attaches to the crystal.
- The acetic acid is used in the food industry and in the households (vinegar); for fixation of photographic films; to remove calcium deposits from cranes and boilers; to treat a jellyfish sting; as a preservative for silage, etc.
- The glacial acetic acid is used in the production of terephthalic acid, propylene terephthalate, synthetic camphor, aniline, etc. etc.
- The acetic acid is not hazardous in low concentrations. The glacial acetic acid is corrosive and its vapors irritate the eyes and nose. In contact with eyes and skin, it can lead to injuries.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
4 Comments
Leave a Response
References :
[0]Hoffman, R.. Organic Chemistry (2nd Edition). Hoboken: John Wiley & Sons, Inc. 2004. Print.
[1]Hoffman, R.. Organic Chemistry (2nd Edition). Hoboken: John Wiley & Sons, Inc. 2004. Print.
[2]Kirkova, E. General Chemistry. Sofia: Kliment Ohridski. 2002. Print.
[3]Petrov, G. Organic Chemistry. Sofia: Kliment Ohridski. 2006. Print.
[4]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/f/f8/Acetic_acid_2.svg/640px-Acetic_acid_2.svg.png
[5]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/1/1e/Acetic_acid_chemical_structure.png/522px-Acetic_acid_chemical_structure.png

Nice artical . Very useful.
Thanks
nice, this information is very helpful.
Very informative , educational now I know how to make vinegar and clean my furniture.
is the structure of acetic acid and glacial acetic acid the same?