Difference Between Acetone and Acetic Acid
Acetone is a chemical with the formula CH3COCH3, which has a fruity smell. Acetic acid is a chemical with the formula CH3COOH, which has a vinegar-like smell.
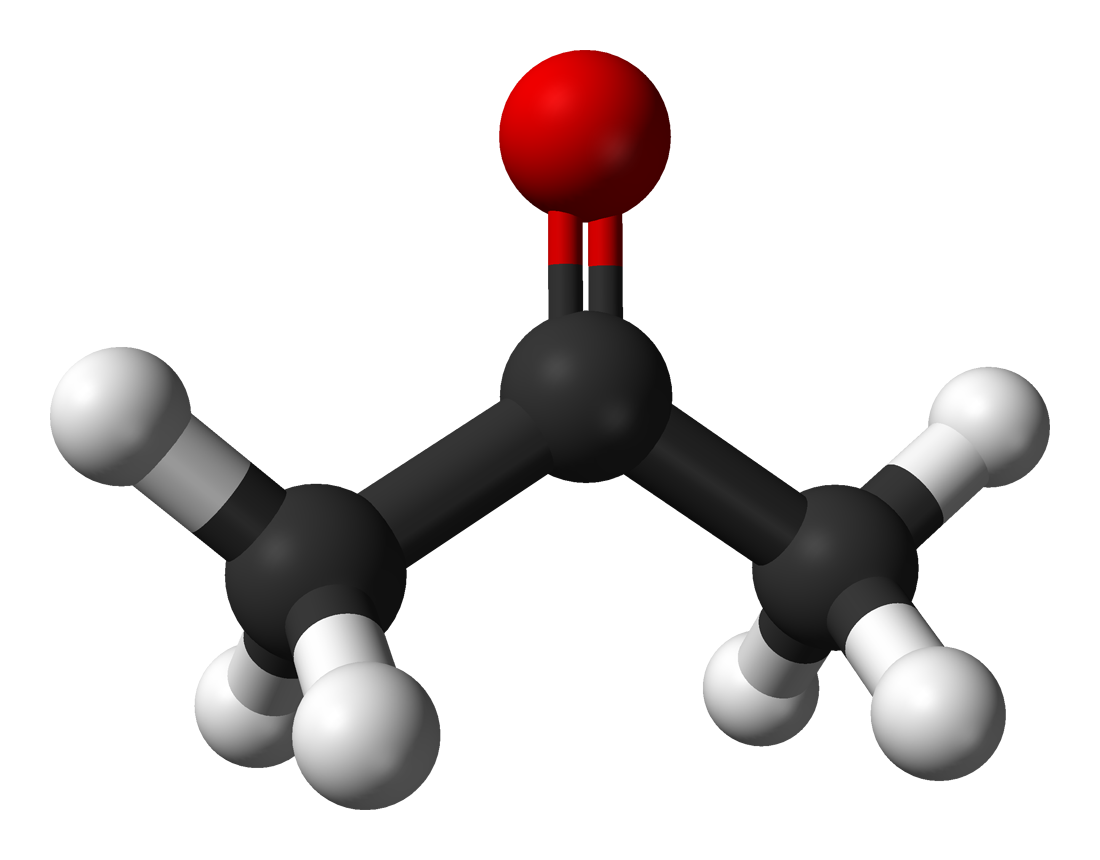
What is Acetone?
Definition:
Acetone is a chemical that has the formula CH3COCH3 and is classified as a ketone.
Properties:
Acetone is known as a ketone which occurs as a colorless liquid which has a smell that is reminiscent of fruit. It has a molecular weight of 58.08 g/mol, and it is also known to be a flammable substance.
Formation:
The cumene hydroperoxide method is how acetone is created in the laboratory. However, acetone is also generated in the human body during the catabolism of lipids and formation of ketones. This can occur with people who have poorly managed diabetes who start to break down fats and end up generating ketones. It is formed in small amounts in healthy people as well as in other living organisms.
Uses:
Acetone can be used as a solvent to remove polish from nails, and in fact, it is commonly sold in stores for this very purpose. It is a good solvent, in general, which is also why it is often used to remove ink or paint. In addition, acetone is used industrially to help make materials such as fibers. It does have some bacteriostatic attributes as well helping to prevent the development of microbes on surfaces, which means it can be used to disinfect surfaces.
Safety:
There are some potential dangers with acetone since it does have flammable properties. It also releases vapors which can be irritating to the mucous membranes and also the skin. It is particularly important for people using large amounts, such as in industry, to be cautious.
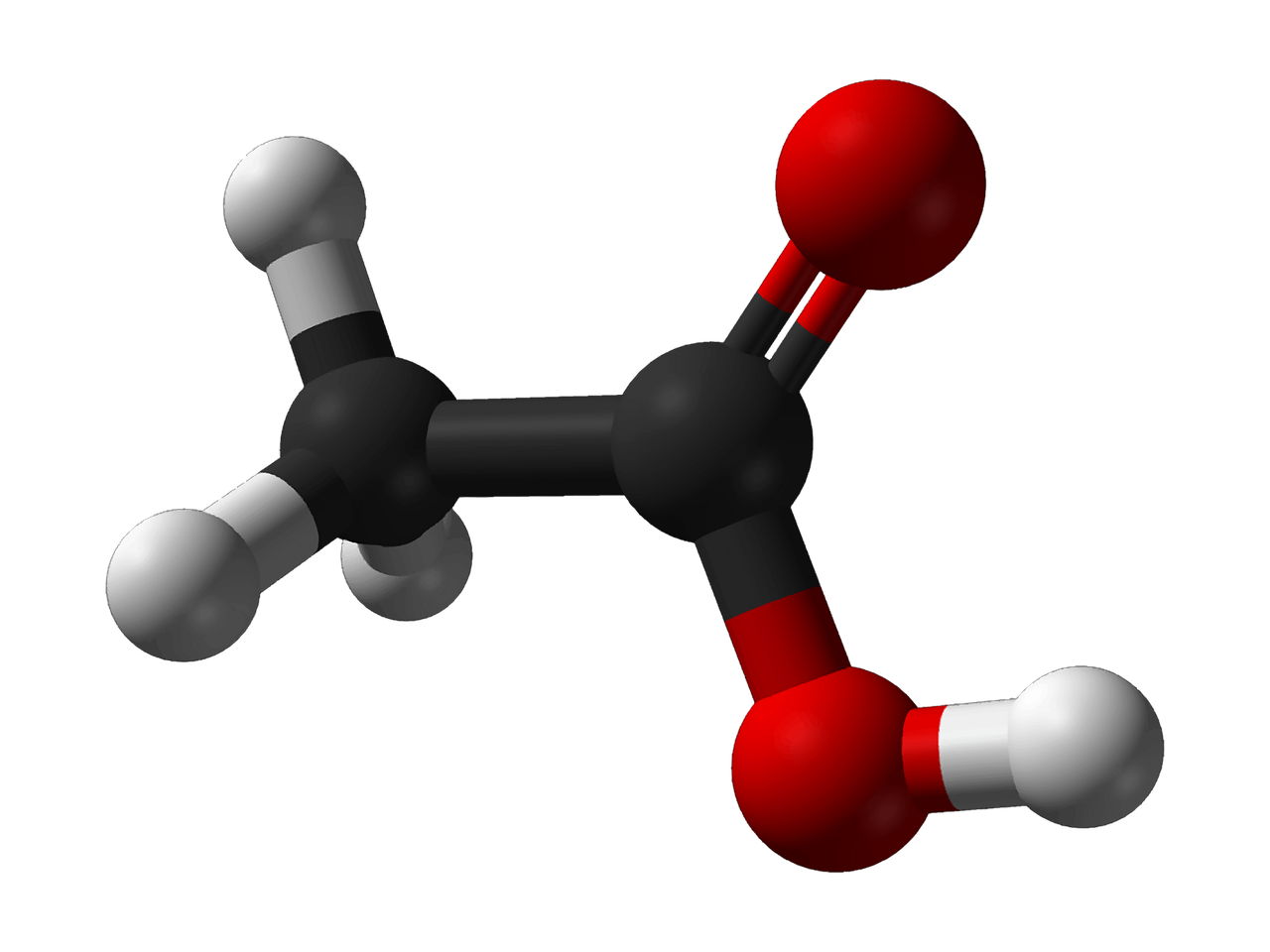
What is Acetic Acid?
Definition:
Acetic acid is a chemical substance that is classified as a carboxylic acid which has the formula CH3COOH or C2H4O2.
Properties:
Acetic acid has a molecular weight of 60.052 g/mol, and is very acidic. In fact, in a 1M solution the pH of acetic acid is 2.4. Acetic acid is a liquid which has no color but has an easily recognizable vinegar-type of smell. This chemical is also hydrophilic and is able to be used to break down both nonpolar and polar molecules, which is advantageous for a solvent. The acetic acid molecule consists of a methyl group bonded to a carboxyl group.
Formation:
Acetobacter bacteria can be used to make acetic acid through a process of fermentation, and the substance can be made artificially in the laboratory through chemical reactions. Acetic acid can be made by reacting methanol with carbon dioxide, and also by oxidation reactions of the chemical substance acetaldehyde.
Uses:
In industry, acetic acid can be used in the process in which polymers are made, for example, the manufacturing of soda bottles. Acetic acid can also be used to make certain types of glue. It is also a useful additive that can be used in food for adding some acidity to a product, and does make up about 9% of commercial vinegar.
Safety:
If a person is not careful, acetic acid can be dangerous because it is a volatile compound and can be corrosive.
Difference between Acetone and Acetic Acid?
Definition
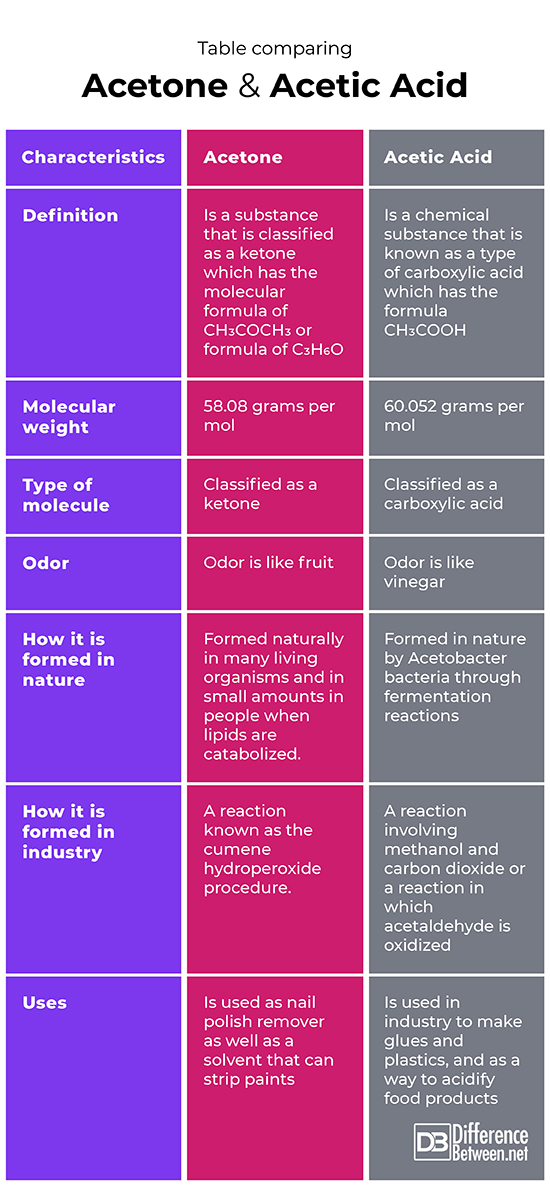
Acetone is a chemical that is a type of ketone, which has the formula CH3COCH3. Acetic acid is a chemical that is a type of carboxylic acid that has the formula CH3COOH or C2H4O2.
Molecular weight
The molecular weight of acetone is 58.08 grams per mol. The molecular weight of acetic acid is 60.052 grams per mol.
Type of molecule
Acetone is classified as a type of molecule called a ketone. Acetic acid is classified as a type of molecule called carboxylic acid.
Odor
The odor of acetone is like that of a fruit. The odor of acetic acid is like that of vinegar.
How it is formed in nature
Acetone is made in many living organisms and is also formed in people when lipids (fats) are being broken down. Acetic acid is made in some living organisms such as Acetobacter bacteria by the process of fermentation.
How it is formed in industry
Acetone is formed artificially by a process called the cumene hydroperoxide procedure. Acetic acid is formed artificially by oxidation of acetaldehyde or by a process that combines methanol and carbon dioxide.
Uses
Acetone is used as nail polish remover and it is used in industrial applications as a solvent that can strip paints. Acetic acid is used in industry to make certain plastic products and glue products, and as a way to make food items more acidic.
Table comparing Acetone and Acetic Acid
Summary of Acetone vs. Acetic Acid
- Acetone is classified as a ketone molecule while acetic acid is classified as a carboxylic acid.
- Both acetone and acetic acid are made in nature and are used in industry to make products.
- Acetone can be used to remove nail polish and to strip paint.
- Acetic acid is a component of household vinegar, and it is used to make various industrial products.
- Difference Between Rumination and Regurgitation - June 13, 2024
- Difference Between Pyelectasis and Hydronephrosis - June 4, 2024
- Difference Between Cellulitis and Erysipelas - June 1, 2024
Search DifferenceBetween.net :
1 Comment
Leave a Response
References :
[0]Image credit: https://pixabay.com/de/vectors/essigs%C3%A4ure-s%C3%A4ure-chemische-chemie-1299149/
[1]Image credit: https://en.wikipedia.org/wiki/File:Acetone-3D-balls.png
[2]Dagley, S., E. A. Dawes, and G. A. Morrison. "Inhibition of growth of Aerobacter aerogenes: the mode of action of phenols, alcohols, acetone, and ethyl acetate." Journal of Bacteriology 60.4 (1950): 369.
[3]US National Library of Medicine. "Acetic acid." NIH, 2019, https://pubchem.ncbi.nlm.nih.gov/compound/Acetic-acid

Remarkable