Difference between Ethanol and Ethanoic Acids
What is Ethanol?
Ethanol is also referred to as ethyl alcohol and has the molecular formula C2H5OH.
It is comprised of an ethyl group (C2H5) and a hydroxyl group (OH).
When in a pure state, ethanol has no color, it is flammable, and has a boiling point of 78.5 °C and a pH of 7.33.
It has a strong odor that is similar to a perfume, and it can evaporate quickly (i.e. it is very volatile).
Ethanol is a component of alcohol that people drink. It has a depressing effect on the nervous system and is toxic, particularly in large enough amounts.
When consumed as an alcoholic beverage it is converted by the liver to acetaldehyde and eventually to carbon dioxide and water.
If a person ingests too much alcohol too quickly, it can lead to coma and even death.
Ethanol that is to be consumed by humans in the form of beer, wine or similar alcoholic beverages, are produced as a byproduct of the process of fermentation of a particular substrate. Sugars and ethanol are produced in the process.
Fermentation in which ethanol is produced usually uses yeasts such as Saccharomyces cerevisiae or Schizosaccharomyces.
The type of alcohol produced depends on the substrate, for instance wine is produced from the fermentation of grapes, and beer is often produced from the fermentation of barley.
Ethanol can also be converted into different intermediate products for use in industrial applications such as the manufacturing of plastics.
It can also be used as an additive to motor vehicle gasoline, where it becomes known as gasohol.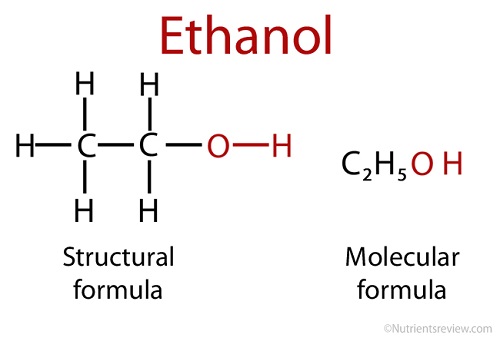
What is Ethanoic Acid?
Ethanoic acid is also referred to as acetic acid. It has the following molecular formula:
C2H4O2 or CH3COOH.
It is considered an acid since the H+ proton can dissociate from the molecule. When a proton is lost, the molecule remaining is referred to as an acetate ion.
The ethanoic acid has a low pH of 2.4 at a 1.0 M concentration. The boiling point of ethanoic acid is between 118 to 119 °C, and it is also a volatile compound.
It has a distinctive vinegar odor and is a colorless liquid.
Ethanol without water present is known as glacial acetic acid.
Ethanoic acid is a carboxylic acid, which as the name implies has a carboxyl group (-COOH), to which is attached a methyl group (CH3).
Ethanoic acid is a hydrophilic solvent that can work on both nonpolar and polar substances, which makes it very useful in industrial applications.
It can be produced by fermentation by Acetobacter bacteria, but also can be produced synthetically.
Ethanoic acid can be formed by combining carbon dioxide with methanol or by oxidizing acetaldehyde.
It is a component of vinegar, where it can form as much as 9% of the vinegar.
It has a corrosive effect on metals, and it can be reduced to form ethanol by nucleophilic acyl substitution.
Ethanoic acid can be used to form polymers such as those used to make plastic soda bottles and even glue for wood.
It is even used as a food additive because it can adjust the acidity of substances.
Difference between Ethanol and Ethanoic Acid
- Ethanol has the molecular formula C2H5OH, while ethanoic acid has the molecular formula C2H4O2.
- Ethanol has an ethyl group while ethanoic acid has a methyl group.
- Ethanoic acid is a carboxylic acid; this is not the case for ethanol.
- Ethanol has a hydroxyl group while ethanoic acid has a carboxyl group.
- Ethanol in a pure state is basic, while ethanoic acid is acidic.
- Ethanoic acid has a boiling point of 118 to 119oC, while ethanol has a boiling point of 78.5o
- Ethanoic acid has a vinegar smell while ethanol has a perfume type of smell.
- Ethanoic acid can be formed using Acetobacter bacteria during fermentation; this is not the case with ethanol.
- Ethanol usually is formed using yeasts such as Saccharomyces cerevisiae or Schizosaccharomyces during fermentation; this is not the case with ethanoic acid.
- Ethanol is used to make alcoholic beverages while ethanoic acid is used to make vinegar.
- Ethanol is also referred to as ethyl alcohol while ethanoic acid is also referred to as acetic acid.
Table comparing Ethanol and Ethanoic Acid
| ETHANOL | ETHANOIC ACID |
| Molecular formula is: C2H5OH | Molecular formula is: C2H4O2. |
| Has an ethyl group present | Has a methyl group present |
| Is not a carboxylic acid | Is a carboxylic acid |
| Has a hydroxyl group present | Has a carboxyl group present |
| Basic in pure state | Acidic in pure state |
| Boiling point of 78.5oC. | Boiling point of 118 to 119oC |
| Perfume smell | Vinegar smell |
| Formed using yeasts such as Saccharomyces cerevisiae or Schizosaccharomyces | Not formed using yeasts such as Saccharomyces cerevisiae or Schizosaccharomyces |
| Not formed using bacteria of Acetobacter spp. | Formed using bacteria of Acetobacter spp. |
| Used to make alcoholic beverages | Used to make vinegar |
| Also referred to as ethyl alcohol | Also referred to as acetic acid |
Summary of Ethanol and Ethanoic Acid
- Ethanol is a colorless flammable substance with the formula C2H5OH, and can be produced by fermentation of various substrates by yeast.
- Ethanoic acid is also colorless and has the formula C2H4O2or CH3 It is a type of carboxylic acid, hence is a weak acid.
- Ethanol has a lower boiling point than ethanoic acid, and has a higher pH.
- Ethanol is a component of alcoholic beverages; the type of alcohol that is formed depends on the type of substrate that is fermented.
- Ethanoic acid is the main component of vinegar, and is also often added to products since it can regulate the acidity.
- Ethanoic acid can also be produced by a fermentation process, but the bacteria Acetobacter is generally used.
- Both ethanol and ethanoic acid can also be synthetically produced via chemical reactions.
- Ethanol can be used in the manufacturing of plastics and as a gasoline additive.
- Ethanoic acid can also be used in the manufacturing of plastics such as plastic bottles, and is often used as a food additive to regulate acidity.
- Difference Between Rumination and Regurgitation - June 13, 2024
- Difference Between Pyelectasis and Hydronephrosis - June 4, 2024
- Difference Between Cellulitis and Erysipelas - June 1, 2024
Search DifferenceBetween.net :
2 Comments
Leave a Response
References :
[0]Encyclopedia Britannica. “Ethyl-alcohol”. Science. Encyclopedia Britannica, 2017, https://www.britannica.com/science/ethyl-alcohol
[1]New World Encyclopedia. "Ethanol." New World Encyclopedia. New World Encyclopedia, 2017, http://www.newworldencyclopedia.org/entry/Ethanol
[2]New World Encyclopedia. "Acetic acid." New World Encyclopedia. New World Encyclopedia, 2017, http://www.newworldencyclopedia.org/entry/Acetic_acid
[3]"Image Credit: http://philschatz.com/chemistry-book/contents/m51002.html"

IN ETHANOL A SINGLE OXYGEN IS BONDED TO CARBON ATOM WHERE THEIR IS SIX MOLECLES OF HYDROGEN BONDED TO TWO CARBON ATOM, SEEN THE CASE IN ETHANOIC ACID THERE TWO OXYGEN AND TWO CARBON ATOMS WHILE LIMITED NUMBERS OF HYDROGEN WHICH ARE FOUR. THE EXCESS OXYGEN AND LIMITED NUMBER OF HYDROGEN MAKES ETHANOL TO BECOMES ETHANOIC ACID.
ETHANOL IS A CONSUMMABLE SUBSTANCE,BUT NOT IN THE CASE OF ETHANOIC ACID. ETHANOL={C2H5OH}. ETHANOIC ACID ={C2H3OOH}
why is the person above me shouting