Difference Between Primary Cell and Secondary Cell
The battery, or serial – parallel combination of electrochemical cells, is an energy storing device that is still extensively in use today. The basic division of batteries according to their usage refers to their ability to be charged.
So there are primary cells – that cannot be charged and secondary (rechargeable) cells.
What is Primary Cell?
Primary cells are those that cannot be recharged and needs to be discarded after the expiration of their lifetime. If the electrolyte is not in liquid form, we are talking about dry cells.
Primary cells usually have high energy density, capacity, are slowly to discharge, easy to use and not excessively expensive. Alkaline are probably most commonly used primary batteries.
They usually have zinc anode, carbon cathode and electrolyte. The voltage curve for discharging alkaline batteries is very steep (almost linear).
When the battery empties, its voltage drops almost linearly. Such cells are therefore not suitable for digital cameras, as they require relatively high voltage for their operation. Alkaline battery is therefore showed as “empty” after a few hours of using it, although in reality it isn’t.
Most of the primary cells are comfortable, always available and environmentally friendly. They also have extremely high energy density.
Only in recent years, the rechargeable cells have reached the density of primary cells, but conventional alkaline batteries produce almost 50% more energy than a comparable Li-Ion secondary cell.
These cells continuously charge and supply all kinds of devices, from basic, all known devices to specialized devices and applications. Primary cells are most commonly used in wristwatches, remote controls, children’s toys and non-demanding entertainment electronics. They are also used wherever the charging is impractical or impossible, in cases of military and rescue techniques, in difficult to access control stations, and the like.
Because of low prices, they are especially suitable where the power requirements are not very high, where the devices do not require a high level of energy for their operation, and need just a constant voltage.
What is Secondary Cell?
With the rise of portable devices such as laptops, smartphones or MP3 players, there is a growing demand for good batteries that we will not have to change every couple of days. And here we come to the need of rechargeable (secondary) cells.
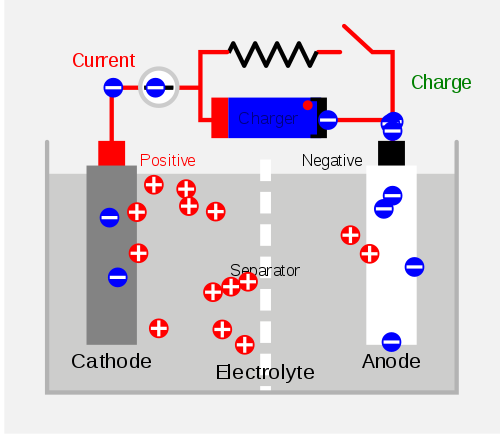
The principle of their work is actually the same – electricity is generated through a chemical reaction involving anode, cathodes and electrolytes, but the difference is in the chemical composition of the cells contained in the batteries.
Here we have the case that the chemical reaction is reversible. When the battery “consumes” (or when the negatively charged ions go to the positive side of the battery), the battery is charged. By connecting a secondary cell to an external source of electrical power (e.g., electricity), the opposite process occurs – the negatively charged ions return to the negative side of the battery and can be used again.
The most commonly used secondary batteries on the market are: lithium-ion (LiOn), nickel-metal hydride (NiMH), and nickel-cadmium (NiCd). When talking about secondary batteries, we must say that they are not all equal. NiCd (nickel cadmium) were the first secondary batteries that were used everywhere in the world, but they had one small problem – “memory effect”.
The memory effect means that you have to refill and empty them every time, otherwise they will lose their capacity quickly. This has led to a situation where people are switching to Nickel–metal hydride (NiMH). They had somewhat greater capacity and did not “suffer” from the memory effect, but their lifespan was short – you could fill them up and empty them around 100 times.
And finally, the most popular LiOn batteries are used today, which have proved to be the best variant. Perhaps their capacity is somewhat smaller, but the technology of making them is simpler than the ones previously mentioned, they are smaller, easier and have a cycle of 1000 charging and discharging.
Difference Between Primary and Secondary Cells
-
Design of Primary and Secondary Cells
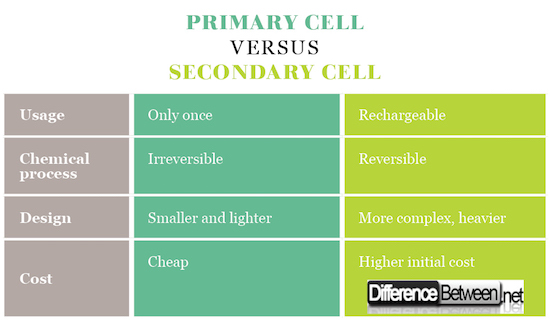
Primary cells are most often “dry cells” – regarding the technology of making them. It’s because there are no fluids in the battery, but the cells are full of paste that allows the movement of the ions, but prevents their spilling. Secondary cells use the other two types of cells – wet cells (liquid, flooded cells) and molten salt (liquid cells with a slightly different composition).
-
Specifications of Primary and Secondary Cells
Primary cells have high internal resistance, irreversible chemical reaction, higher capacity, are typically smaller and lighter, and are cheaper overall. Secondary cells have lower internal resistance, need to be charged, have reversible chemical reactions and are more complex and expensive.
-
Application of Primary and Secondary Cells
Primary cells are used in devices of a need of small but constant current – clocks, toys, safety equipment and so on. Secondary cells are used in portable devices – laptops, mobile phones, mp3 players, tablets etc.
Primary vs. Secondary Cell : Comparison Table
Summary of Primary and Secondary Cells
- Primary cells are capable of producing electric current at the time of the genesis. They are also called disposable batteries, as they are intended for single use and discarding. These are the most commonly used cells in portable devices that do not require high voltage. In principle, primary batteries cannot be reliably filled repeatedly because chemical reactions are not reversible and the materials used are unlikely to return to their primary state.
- Secondary cells must be charged before use. Also called rechargeable batteries, they can be recharged by bringing them an electric current, which reverses the course of the chemical reaction that occurs during the use of the battery.
- Difference Between Thermodynamics and Kinetics - June 24, 2018
- Difference Between Welding and Soldering - June 24, 2018
- Difference Between Additive Colors and Subtractive Colors - June 20, 2018
Search DifferenceBetween.net :
3 Comments
Leave a Response
References :
[0]Reddy, T. B. “Handbook of Batteries.”, Third edition, NY: McGraw-Hill, 2002. Print
[1]Crompton, T. R. “Battery Reference Book”, Third edition, Oxford: Newnes, Oxford, 2000. Print
[2]Linden, D., Reddy, T. B. “Handbook of Batteries”, Third edition, NY: McGraw Hill, 2001. Print
[3]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/8/88/Secondary_Cell_Diagram.svg/500px-Secondary_Cell_Diagram.svg.png
[4]Image credit: https://pixabay.com/en/batteries-loading-icons-set-flat-1379208/

Very nice
Thank you so much
Primary and secondary cell mai antar
Ok, BUT WHERE ARE THE SPECIFIC RESISTANCES OF EACH?!