Difference Between Radon and Radium
What is Radon?
Radon is a radioactive gas that is a byproduct of the decay of radium. It is part of the decay series in which uranium decays into multiple elements until reaching the stable element, lead. Radon decays into polonium and alpha particles. The longest-lived isotope of radon is radon-222 which has a half-life of 3.8 days.
Discovery of Radon
Radon was first detected in 1899 by Pierre and Marie Curie as a gas being released by the decay of radium. At the same time, the physicist Ernst Rutherford discovered a radioactive gas being released by thorium in his experiments. In 1900, it was officially discovered by Friedrich Ernst Dorn a scientist in Halle, Germany.
Impact on health
Since its discovery, it has been found to be a major health hazard. It is strongly associated with occurrences of lung cancer. Radon is inhaled by those exposed to it. The abundance of radon depends on the local geology including the abundance of uranium or thorium in the soil. As radon is inhaled, it also decays into polonium, another radioactive element, which can increase the amount of radioactive material in the body. This can result in the production of cancerous cells.
While radon can be related to causing cancer, it has also been used in the past to treat cancer. In the 20th century, radon gas would be injected into tumors and cancerous cells to destroy them. Although radon is short-lived, it is common enough that it makes up a noticeable part of the background radiation of Earth.
Impacts on the history of life on Earth
Because of this, it has been suggested that it may have played an important role in evolution because of the mutagenic effects of the radioactive gas. Regions with higher radon content in the country rock may have led to more mutations in the local plant, animal, and microbial life, leading to more mutations and thus more evolution among those populations.
What is Radium?
Radium is a metal that is part of the uranium-lead decay series. It is known to be highly radioactive. It was first discovered in 1898 by Pierre and Marie Curie in an ore of uranium. They identified the element because it had the capacity to glow. The metal in its pure form was first produced in 1911 by Marie Curie and one of her colleagues. The name of the element comes from the Latin word for “ray,” referring to its radioactivity.
Properties
Radium is a silvery, soft metal. It can glow in the dark in its pure form because of its radioactivity. It is also the 84th most common element in Earth’s crust, having an abundance of one part per trillion. It is also the heaviest of the alkaline earth metals and can combine with most nonmetals including nitrogen and oxygen to create rare molecules. The isotope of radium with the longest half-life is radium-226 which has a half-life of about 1600 years.
Uses of radium
Radium, because it can glow was once used to make luminous paints. For example, it was once used on clocks that were designed to be visible in the dark and was even used in toothpaste. This was before it was discovered to be highly radioactive. In some cases, radium has been used to treat prostate cancer that has spread to bone tissue. This is because of the similarity between radium and calcium and the fact that bones contain calcium.
Health hazards
The degree of radioactivity in the element radium is demonstrated by the fact that Marie Curie’s notebooks that she used to study radium are still too radioactive to be safely handled. Because of this, radium can easily increase the occurrence of cancer, blood problems such as anemia, eye problems such as cataracts, and dental problems.
Workers likely to experience more exposure to radium include miners, particularly uranium miners. Water from wells and air near factories using fossil fuels also have higher amounts of radium. Because of the abundance of radium in Earth’s crust, humans and other life forms are constantly exposed to non-harmful levels of radiation from the element.
Similarities between Radon and Radium
They are both radioactive and they are both ultimately products of the decay of uranium to lead. They are also both known to be cancer-causing but have also been used to treat cancer. Life on Earth is also exposed to constant, non-harmful levels of radiation from radium and radon because of the relative abundance of both elements in the crust.
Differences between Radon and Radium
There are many similarities but there are also notable differences. These include the following.
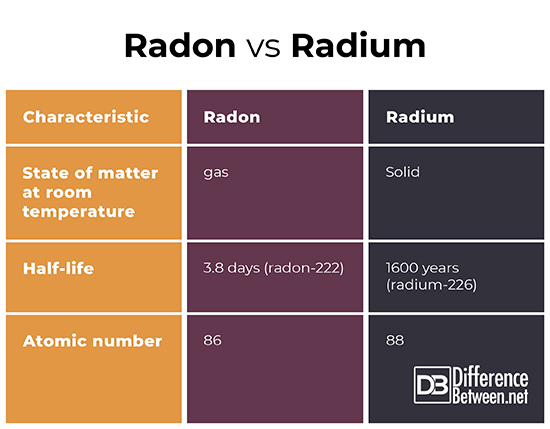
- Radium is a solid at room temperature while radon is a gas at room temperature.
- The longest-lived isotope of Radium has a half-life of 1600 years while the longest-lived isotope of radon has a half-life of only 3.8 days.
- The atomic number of radium is 88 while the atomic number of radon is 86.
Radon vs. Radium
Summary of Radon vs. Radium
Radon is a gas that is radioactive. It is the product the decay of uranium ultimately into its stable daughter product, lead. It is the direct daughter product of radium. It is relatively abundant in Earth’s crust and its distribution depends on the local geology. It is a health hazard and associated with lung cancer. Radium is a metal that is also part of the decay series from uranium to lead. Radium is so radioactive that it can glow and at one point it was used to make luminous paints, though now that is considered too dangerous. Radium occurs throughout Earth’s crust but occurs in unusually high levels in certain locations such as uranium mines and fossil-fuel driven factories. Radon and radium are both radioactive and products of uranium decay. They also both cause cancer and ironically have been used to treat cancer. Nonetheless, they are different in that radon is a gas while radium is a solid at room temperature. Also, the longest-lived isotope of radium has a half-life of about 1600 years while the longest-lived isotope of radon only has a half-life of 3.8 days.
- Difference Between Environmental Performance Index and Development - November 24, 2023
- Difference Between Environmental Intervention and Development - November 8, 2023
- Difference Between Eco Efficiency and Eco Effectiveness - September 18, 2023
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://commons.wikimedia.org/wiki/File:Electron_shell_088_Radium.svg
[1]Image credit: https://sw.m.wikipedia.org/wiki/Picha:Electron_shell_086_Radon.svg
[2]Ross, Rachel. 2016. Facts About Radium. Live Science https://www.livescience.com/39623-facts-about-radium.html
[3]National Research Council. Health effects of exposure to radon: BEIR VI. Vol. 6. National Academies Press, 1999.
[4]Hopke, Philip K. "Radon and its decay products: Occurrence, properties, and health effects." (1987).
[5]“Radon.” National Institute of Health ad Environmental Sciences. Available at: https://www.niehs.nih.gov/health/topics/agents/radon/index.cfm
[6]“What is Radon?” United States Geological Survey Environmental Health. Available at: https://www.usgs.gov/faqs/what-radon?qt-news_science_products=0#qt-news_science_products
[7]Elements 1-112, 114, 116 and 117 © John Emsley 2012. Elements 113, 115, 117 and 118 © Royal Society of Chemistry 2017
[8]John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements, Oxford University Press, New York, 2nd Edition, 2011.
[9]W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
[10]“Radium CAS # 7440-14-4.” 1999. Agency for Toxic Substances and Disease Registry. Available at: https://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=790&tid=154