Difference Between Bioburden and Microbial Limit Test
The field of pharmaceutical microbiology has evolved over the years, owing to the recent technological advances, accompanied by the publication of new and harmonized compendial methods. This is why it’s essential for microbiologists responsible for monitoring the microbial quality of pharmaceutical products to keep up with the changes around. The microbiological quality of the finished products is determined by the quality of the starting materials, which are, in most cases, non-sterile materials or products. Such products are either used to manufacture more complex pharmaceutical products or used in the preliminary stages of what will become sterile products.
Although, most of the products released do not require sterilization, some products are tested for the presence of objectionable microorganisms. The presence of certain microorganisms in some non-sterile products may affect the therapeutic characteristics of the product and in some cases, the health of the patient. So, for those products, additional testing is required for indicator microorganisms. Microbial examination or testing of those non-sterile products is performed by “Microbial Limits Test”, which determines the bioburden of the product. Bioburden refers to the number of microorganisms on a surface or in a solution that has not been sterilized.
What is a Bioburden Test?
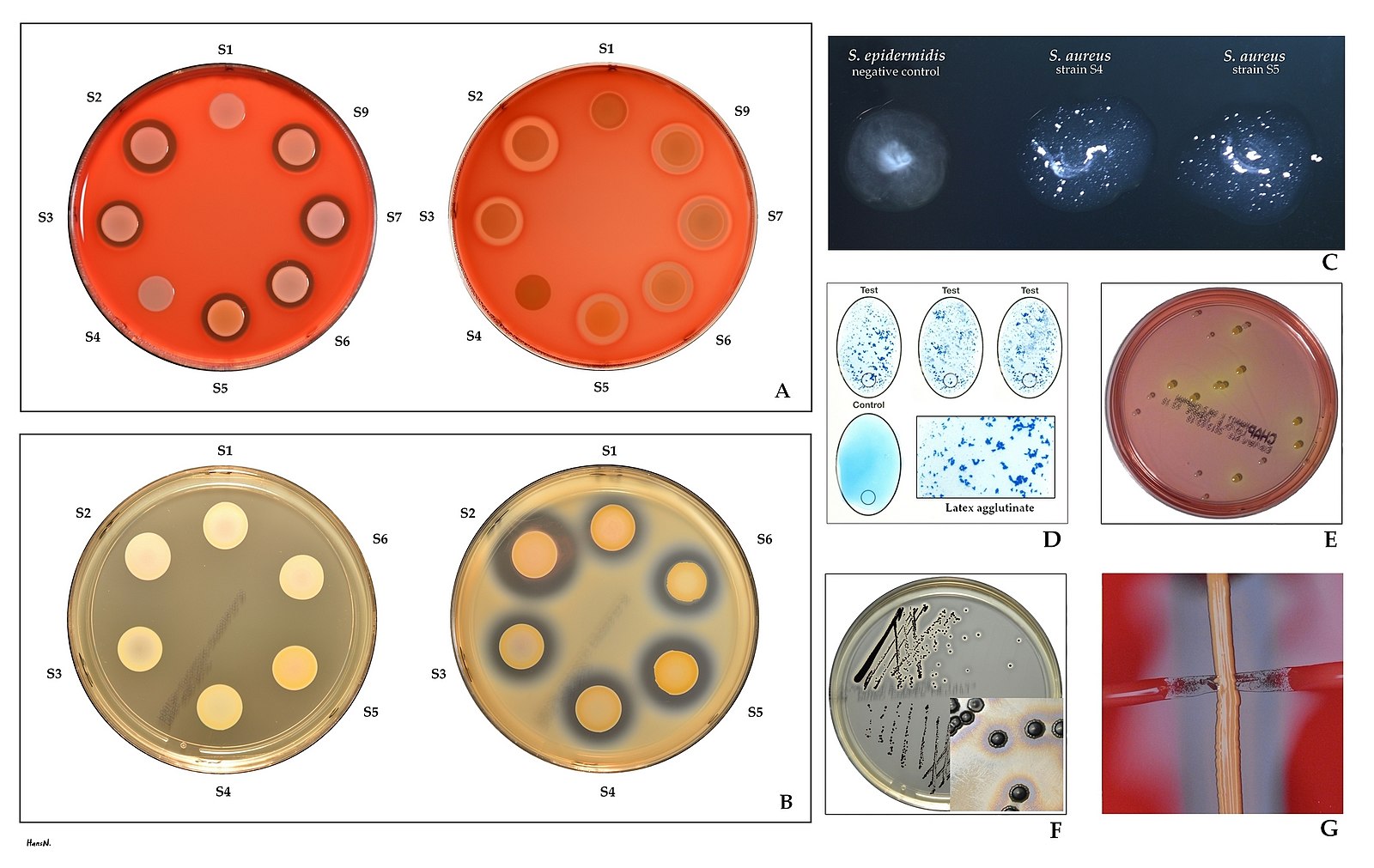
Bioburden refers to the number of microorganisms that can be detected on an item or surface or in a solution. It is necessary to know the initial number of microorganisms for selection of parameters for any method intended to kill microorganisms. This initial number is called ‘bioburden’ or ‘bioload’. The microbial recovery method used depends on the type of material being evaluated. The need for a thorough analysis of bioburden is an area often underestimated in the validation process. A bioburden test is performed for quality control purposes to measure the microbial contamination levels on or in a product. It is a total viable count (TVC) test to estimate the viable aerobic mesophilic microorganisms in products or articles not purported to be sterile. Knowledge of the bioburden involves knowing both the population of microorganisms on the device and the nature of the resistance of the microorganisms.
What is a Microbial Limit Test?
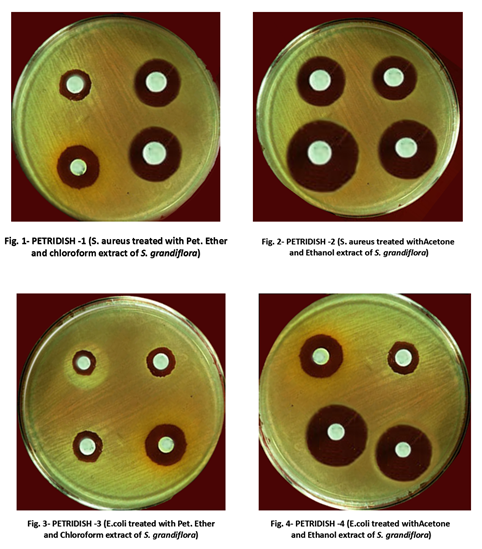
Microbial Limit Test is the ‘Test for Specified Microorganisms’ designed to determine the qualitative and quantitative estimation of viable aerobic microorganisms in pharmaceutical products of natural or biological origin, raw materials, and finished products. Raw materials are those products that can be used for further processing or to aid in such processing. Non-sterile products are tested for viable microorganisms for detection of pathogens and total viable counts. The microbiological quality of non-pharmaceutical or cosmetic products can be controlled by using estimation of the total viable count and detecting the presence of specific microbial species in those substances. The microbial limit test comprises the total aerobic microbial count (TAMC), the total combined yeasts and molds count (TYMC), and tests for indicator organisms. The aim of microbial limit test is pretty simple – to exclude any microorganisms that may subsequently result in deterioration of the product or may harm the patient.
Difference between Bioburden and Microbial Limit Test
Test
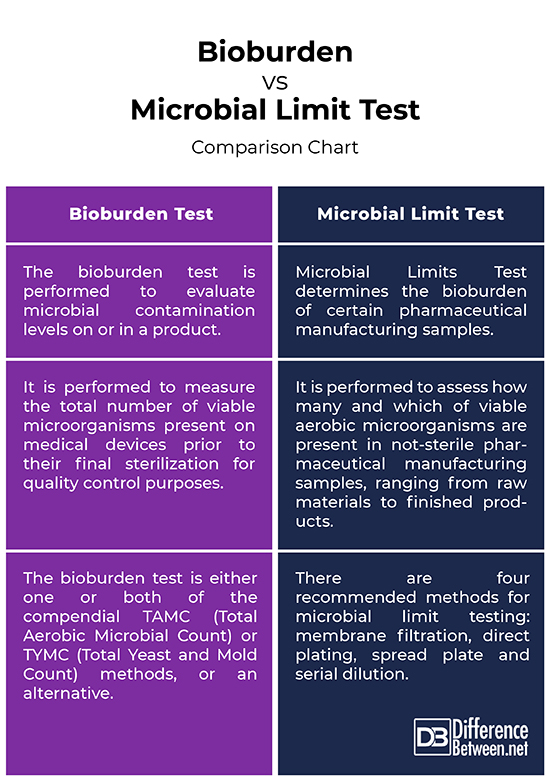
– Microbial Limits Test determines the bioburden of certain pharmaceutical manufacturing samples for quality control purposes. Bioburden refers to the number of microorganisms on a surface or in a solution that has not been sterilized. The bioburden test is performed to evaluate microbial contamination levels on or in a product. Knowledge of the bioburden involves knowing both the population of microorganisms on the device and the nature of the resistance of the microorganisms. Microbial limits test provides the information necessary to conduct bioburden counts by quantitative estimation of viable aerobic microorganisms in pharmaceutical articles, raw materials and finished products. Bioburden test is also referred to as total viable count.
Method
– Bioburden test determines the number of microorganisms on an item or surface or in a solution. A sustainability test needs to be performed first before the actual bioburden test, which is to ensure that the bioburden test is effective in recovering microorganisms present on the device. The bioburden test is either one or both of the compendial TAMC (Total Aerobic Microbial Count) or TYMC (Total Yeast and Mold Count) methods, or an alternative. For microbial assessing and examination, there are four recommended methods: membrane filtration, direct plating, spread plate and serial dilution.
Objective
– The objective of bioburden test is to measure the total number of viable microorganisms present on medical devices prior to their final sterilization and then use the number to determine the most appropriate parameters for their final sterilization before being put to use. Non-sterile products are tested for viable microorganisms for detection of pathogens and total viable counts. The microbial limit test (MLT) is performed to assess the qualitative and quantitative estimation of viable aerobic microorganisms present in not-sterile pharmaceutical manufacturing samples, ranging from raw materials to finished products.
Bioburden vs. Microbial Limit Test: Comparison Chart
Summary
In a nutshell, non-sterile products are tested for viable microorganisms for detection of pathogens and total viable counts. While most of the products released do not require sterilization, some products are tested for the presence of objectionable microorganisms. The presence of certain microorganisms in certain non-sterile products may affect the therapeutic characteristics of the product. Microbial examination or testing of those non-sterile products is performed by “Microbial Limits Test”, which determines the bioburden of the product, which includes both raw materials and finished products.
- Difference Between Caucus and Primary - June 18, 2024
- Difference Between PPO and POS - May 30, 2024
- Difference Between RFID and NFC - May 28, 2024
Search DifferenceBetween.net :
1 Comment
Leave a Response
References :
[0]Ahuja, Satinder and Stephen Scypinski. Handbook of Modern Pharmaceutical Analysis. San Diego, California: Academic Press, 2001. Print
[1]Booth, Anne. Sterilization of Medical Devices. Abingdon, United Kingdom: Routledge, 2018. Print
[2]Clontz, Lucia. Microbial Limit and Bioburden Tests: Validation Approaches and Global Requirements (Second Edition). Boca Raton, Florida: CRC Press, 2008. Print
[3]Sandle, Tim. Pharmaceutical Microbiology: Essentials for Quality Assurance and Quality Control. Cambridge, United Kingdom: Woodhead Publishing, 2015. Print
[4]Image credit: https://commons.wikimedia.org/wiki/File:Zone_of_inhibition_by_microorganism_bks.png
[5]Image credit: https://commons.wikimedia.org/wiki/File:Staphylococcus_aureus_identification.jpg

Why we should perform sterility test in closed condition