Difference Between Acetone and Polystyrene
Acetone is a mobile liquid with a pungent odor and a peppermint-like taste. In fact, it smells much like the related chemical methyl ethyl ketone. Acetone is a strong solvent for the plastic, but evaporates rapidly. Nearly half of all the acetone manufactured is used for making acrylic plastic. Polystyrene, on the other hand, is a choice of material for commercial packaging because it’s lightweight and is insensitive to moisture. It can be solid or foamed. Polystyrene foams are commonly used for its insulating properties in the construction industry. Let’s take a good look at the two compounds and see how they compare.

What is Acetone?
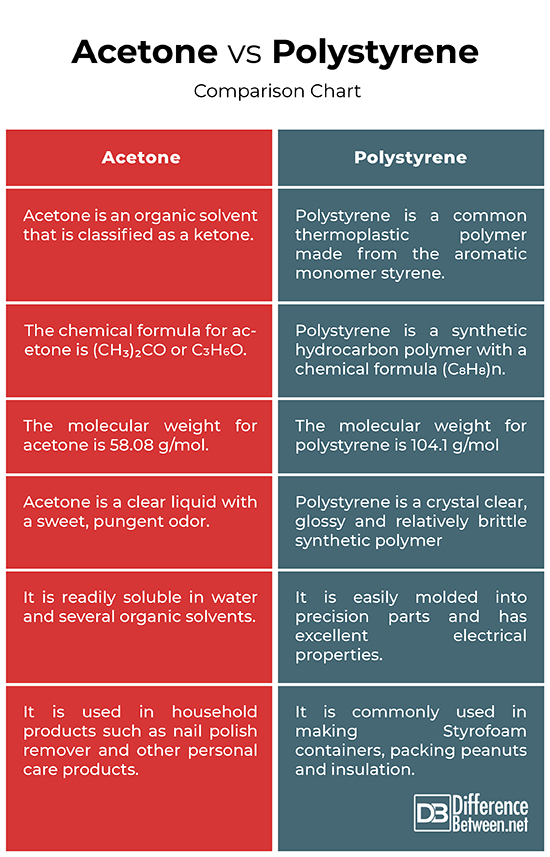
Acetone is an organic solvent that is classified as a ketone. It is a colorless, flammable liquid that is primarily used in industrial applications such as manufacturing of plastics. Acetone is also used in household products such as nail polish remover and other personal care products. Because of the presence of carbon atoms it is an organic compound with the chemical formula (CH₃)₂CO. It is also used as a solvent for paint, varnish, inks, lacquers, and adhesives. Acetone is a strong solvent for the plastic, but evaporates rapidly. Acetone and several other ketones are produced in the liver as a result of fat metabolism. Additionally, it is used as a chemical intermediate in the production of pharmaceuticals, plastics and resins. It is also used to remove oil stains from the walls.

What is Polystyrene?
Polystyrene is a synthetic resin and a commonly used type of plastic used to make Styrofoam containers, packing peanuts and insulation. It is a common thermoplastic polymer made from the aromatic monomer styrene with good formability. Styrene is an important feedstock in a variety of polymer products and of the total amount of styrene produced, almost 50% is used to make polystyrene. The general-purpose polystyrene is hard, stiff and rather brittle. It is widely used in automotive, electrical and electronic connector systems. The most common use of polystyrene is for commercial packaging. It is an inexpensive plastic available in both clear and textured sheet form to provide lightweight double glazing in place of glass. In its solid form, it is used in making medical devices such as test tubes or petri dishes.
Difference between Acetone and Polystyrene
Basics of Acetone vs. Polystyrene
Acetone is an organic solvent that is classified as a ketone and is an important solvent typically used for laboratory and industrial applications. It is a colorless, flammable liquid commonly found in personal care products such as a nail polish remover. Also known as dimethyl ketone, acetone is the simplest and the most vital of all the aliphatic ketones.
Polystyrene, on the other hand, is a common thermoplastic polymer made from the aromatic monomer styrene with good formability. Styrene is an important feedstock in a variety of polymer products.
Chemical Properties in Acetone and Polystyrene
The chemical formula for acetone is (CH₃)₂CO or C3H6O. It consist of three carbon atoms, six hydrogen atoms, and a single atom of oxygen. The molecular weight for acetone is 58.08 g/mol and its melting point is -95°C and boiling point is 56°C.
Polystyrene is a synthetic hydrocarbon polymer with a chemical formula (C8H8)n. Its molecular weight is 104.1 g/mol and its melting point is 240°C and boiling point is 100°C.
Physical Properties in Acetone vs. Polystyrene
Acetone is a clear liquid with a sweet, pungent odor. It is a colorless, flammable liquid which smells much like the related chemical methyl ethyl ketone and it evaporates and catches fire easily. It is readily soluble in water and several organic solvents. Acetone is naturally produced by the body as a product of metabolism.
Polystyrene, on the other hand, is a crystal clear, glossy and relatively brittle synthetic polymer which usually softens when heated beyond its glass transition temperature. It is insensitive to moisture and resistant to salt solutions, alkaline liquids, and non-oxidizing agents, ketones, esters and ethers. It is easily molded into precision parts and has excellent electrical properties.
Applications of Acetone vs. Polystyrene
Acetone is primarily used as a solvent in products such as paints, varnish, inks, lacquers, and adhesives. It is used as cleaning agents in household products like a nail polish remover and other personal care products. It is commonly used as a chemical intermediate in the production of pharmaceuticals, plastics and resins.
Polystyrene is a commonly used type of plastic typically used in making Styrofoam containers, packing peanuts and insulation. The most common use of polystyrene is for commercial packaging. Most disposable cell culture dishes and plates are in fact made of polystyrene. It has excellent optical clarity and is hard enough to withstand the daily use in incubators.
Acetone vs. Polystyrene: Comparison Chart
Summary of Acetone vs. Polystyrene
In a nutshell, acetone is more like a household product commonly used in nail polish remover and other personal care products. Acetone is a strong solvent for the plastic, but evaporates rapidly. Acetone catches fire easily. Nearly half of the acetone manufactured is used to make acrylic plastic. Polystyrene is yet another type of plastic commonly used in commercial packaging, mostly food service products. Polystyrene is biologically inert, has excellent optical clarity and is hard enough to withstand the daily used in incubators and other cell culture apparatus.
- Difference Between Caucus and Primary - June 18, 2024
- Difference Between PPO and POS - May 30, 2024
- Difference Between RFID and NFC - May 28, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://www.maxpixel.net/Styrofoam-Texture-White-Structure-Polystyrene-549729
[1]Image credit: https://sco.m.wikipedia.org/wiki/File:Sample_of_Acetone.jpg
[2]Peraro, James. Limitations of Test Methods for Plastics, Issue 1369. Pennsylvania, United States: ASTM International, 2000. Print
[3]Pethrick, Richard A. Polymer Yearbook. Boca Raton, Florida: CRC Press, 1991. Print
[4]Harte, John, et al. Toxics A to Z: A Guide to Everyday Pollution Hazards. Berkeley, California: University of California Press, 2013. Print
