Difference Between Bromine and Chlorine
VIIА group of the Periodic system includes the elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At). All of these elements are known under the generic name “halogen elements” and have nonmetallic chemical properties.
What is Bromine?
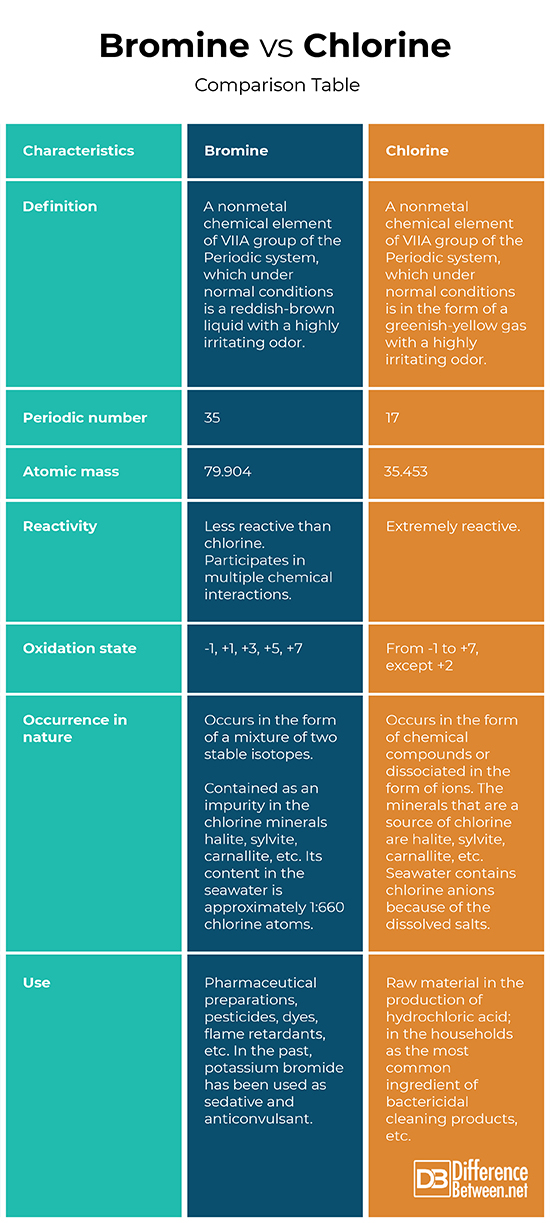
- Bromine (Br) is a nonmetal chemical element of VIIA group of the Periodic system, which under normal conditions is a reddish-brown liquid with a highly irritating odor.
- Bromine is number 35 in the Periodic table and has an atomic mass of 79.904.
- It is less reactive than chlorine. However, under normal conditions, bromine participates in multiple chemical interactions.
- An aqueous solution of molecular bromine (2.8%) is called bromine water and is used in many organic reactions. Generally, the bromine is very well soluble in organic solvents. Often, in the compounds, it exhibits a -1 oxidation state, but can also be in any odd positive oxidation state (+1, +3, +5, +7).
- In nature, bromine occurs in the form of a mixture of two stable isotopes. It is contained as an impurity in the chlorine minerals halite (NaCl – salt), sylvite (KCl), carnallite (potassium magnesium hexahydrate), etc. Its content in the seawater is approximately 1:660 chlorine atoms.
- The use of bromine includes pharmaceutical preparations, pesticides, dyes, flame retardants, etc. In the past, potassium bromide has been used as sedative and anticonvulsant.
What is Chlorine?
- Chlorine (Cl) is a nonmetal chemical element of VIIA group of the Periodic system, which under normal conditions is in the form of a greenish-yellow gas with a highly irritating odor.
- Chlorine is number 17 in the Periodic table and has an atomic mass of 35.453.
- Chlorine is an extremely active chemical element. In chemical interactions, it usually appears to be a strong oxidant and in its chemical compounds is most often of -1 oxidation state. When there is a stronger oxidant in the molecule, the chlorine atoms have a positive oxidation state. For example, in the perchloric acid, the oxidation state of the chlorine atoms reaches +7. Chlorine can be present in all oxidation states from -1 to +7, except +2.
- In nature, sources of chlorine as a simple substance are not known so far and it only occurs in the form of chemical compounds or dissociated in the form of ions. The minerals that are a source of chlorine are halite (NaCl – salt), sylvite (KCl), carnallite (potassium magnesium hexahydrate), etc. The seas and oceans contain chlorine anions because of the dissolved salts. Since the life on Earth comes from the same salty waters, the internal fluid of our organisms also contains an abundance of chlorine ions.
- Chlorine is an important raw material in the production of hydrochloric acid. Its use in the households is quite significant because it is the most common ingredient of bactericidal cleaning products. Chlorine-containing preparations destroy all known bacteria. In the form of radicals, chlorine atoms are extremely reactive, which makes them strong carcinogens.
Difference Between Bromine and Chlorine
Definition
Bromine: Bromine is a nonmetal chemical element of VIIA group of the Periodic system, which under normal conditions is a reddish-brown liquid with a highly irritating odor.
Chlorine: Chlorine is a nonmetal chemical element of VIIA group of the Periodic system, which under normal conditions is in the form of a greenish-yellow gas with a highly irritating odor.
Periodic number
Bromine: Bromine is number 35 in the Periodic table.
Chlorine: Chlorine is number 17 in the Periodic table.
Atomic mass
Bromine: The atomic mass of bromine is 79.904.
Chlorine: The atomic mass of chlorine is 35.453.
Reactivity
Bromine: Bromine is less reactive than chlorine. However, under normal conditions, bromine participates in multiple chemical interactions.
Chlorine: Chlorine is an extremely active chemical element.
Oxidation state
Bromine: Often in the chemical compounds bromine exhibits a -1 oxidation state, but can also be in any odd positive oxidation state (+1, +3, +5, +7).
Chlorine: Chlorine can be present in all oxidation states from -1 to +7, except +2. It is a strong oxidant and in its chemical compounds is most often of -1 oxidation state.
Occurrence in nature
Bromine: In nature, bromine occurs in the form of a mixture of two stable isotopes. It is contained as an impurity in the chlorine minerals halite (NaCl – salt), sylvite (KCl), carnallite (potassium magnesium hexahydrate), etc. Its content in the seawater is approximately 1:660 chlorine atoms.
Chlorine: In nature chlorine only occurs in the form of chemical compounds or dissociated in the form of ions. The minerals that are a source of chlorine are halite (NaCl – salt), sylvite (KCl), carnallite (potassium magnesium hexahydrate), etc. Seawater contains chlorine anions because of the dissolved salts.
Use
Bromine: The use of bromine includes pharmaceutical preparations, pesticides, dyes, flame retardants, sanitizing of pools, etc. In the past, potassium bromide has been used as sedative and anticonvulsant.
Chlorine: Chlorine is used as raw material in the production of hydrochloric acid, in the households as the most common ingredient of bactericidal cleaning products, for sanitizing of pools, etc.
Bromine Vs. Chlorine: Comparison Table
Summary of Bromine Vs. Chlorine
- Bromine is a nonmetal chemical element of VIIA group of the Periodic system, which under normal conditions is a reddish-brown liquid with a highly irritating odor.
- Chlorine is a nonmetal chemical element of VIIA group of the Periodic system, which under normal conditions is in the form of a greenish-yellow gas with a highly irritating odor.
- Bromine is number 35 in the Periodic table and chlorine is number 17.
- The atomic mass of bromine is 79.904, the atomic mass of chlorine is 35.453.
- Bromine is less reactive than chlorine. However, under normal conditions, bromine participates in multiple chemical interactions.
- Often in the chemical compounds bromine exhibits a -1 oxidation state, but it can also be in any odd positive oxidation state (+1, +3, +5, +7). Chlorine can be present in all oxidation states from -1 to +7, except +2.
- In nature, bromine occurs in the form of a mixture of two stable isotopes. Chlorine only occurs in the form of chemical compounds or dissociated in the form of ions.
- The use of bromine includes pharmaceutical preparations, pesticides, dyes, flame retardants, sanitizing of pools, etc. In the past, potassium bromide has been used as sedative and anticonvulsant. Chlorine is used as raw material in the production of hydrochloric acid, in the households as the most common ingredient of bactericidal cleaning products, for sanitizing of pools, etc.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
Leave a Response
References :
[0]De, A. A Text Book of Inorganic Chemistry. New Delhi: New Age Publishers. 2003. Print.
[1]Housecroft, C., A. Sharpe. Inorganic Chemistry. London: Pearson Education Limited. 2012. Print.
[2]Lazarov, D. Inorganic Chemistry. Sofia: St. Kliment Ohridski. 1995. Print.
[3]Image credit: https://en.wikipedia.org/wiki/Bromine#/media/File:Bromine_vial_in_acrylic_cube.jpg
[4]Image credit: https://www.flickr.com/photos/137789813@N06/22925782926