Difference Between Gypsum and Anhydrite
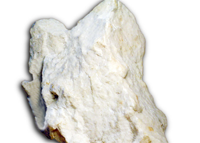
Gypsum, also called hydrated calcium sulphate (CaSO4 · 2H2O) and anhydrite (CaSO4), also called hydrous calcium sulphate are the major minerals in the sedimentary rocks of rock gypsum and rock anhydrite respectively. Gypsum consists of calcium, sulfur and water while anhydrite consists of calcium, sulfur and oxygen. The rocks are commonly referred to as evaporates. Gypsum is monoclinic and usually occurs as twinned tabular crystals although it may also occur as simple. Gypsum also forms fine granular masses, at times coarse. In its typical form, gypsum is colorless or white but if impurities are present then it may be red, brown or orange and it cleaves into plates that can be bent but are not flexible. Gypsum has a soft texture and it can be easily scratched. Its crystals are very flexible and slim crystals can be slightly bent. Sometimes, gypsum forms in sandy places and sand may be trapped inside the crystals when they are forming, causing the gypsum specimen to become brown and opaque. It is a very common mineral and it can be found in numerous localities.
Anhydrite is orthorhombic and does not react with hydrochloric acid. Anhydrite is a hard crystal with a hardness rating of 3.5 and approximate density of 3.0. It is a rare mineral since much of its existing specimen alter to the much more gypsum when altered. Anhydrite usually occurs in arid places forming from the dehydration of gypsum. When exposed to water, anhydrite slowly turns into gypsum. It is sometimes used as an ornamental stone or as a soil conditioner. It has industrial uses too for instance as a drying agent or as a cement additive. Salt domes give the best anhydrite crystals because the domes absorb any underground water thereby preventing it from entering the structure of the Anhydrite, as water would cause it to alter to gypsum. When large deposits of anhydrite are exposed to the earth’s surface, they may alter to gypsum if not immediately collected and covered. Likewise if specimens are collected and kept in moist conditions they may also alter to gypsum. As a recommendation, anhydrite specimens should always be kept in a dry place or should be stored with silica gel, which will absorb the moisture in the air. Anhydrite specimens are quite rare.
Summary:
1. Gypsum is hydrated while Anhydrite does not contain water.
2. Gypsum has a monoclinic crystal form whereas Anhydrite has an orthorhombic crystal structure.
3. Whereas both minerals contain sulfur, Anhydrite contains oxygen while Gypsum does not contain oxygen.
4. Gypsum contains water while Anhydrite does not, instead alters into Gypsum when exposed to water.
- Difference Between Nuts and Bolts - August 2, 2010
- Differences Between Crystal and Gold Silver - August 2, 2010
- Difference Between Crystal Reports and Web Intelligence - August 1, 2010
