Difference Between Ketone and Ester
A ketone has a molecular structure that includes a carbonyl bonded to carbons while an ester has a molecular structure in which a carbonyl is bonded to an alkoxy group.
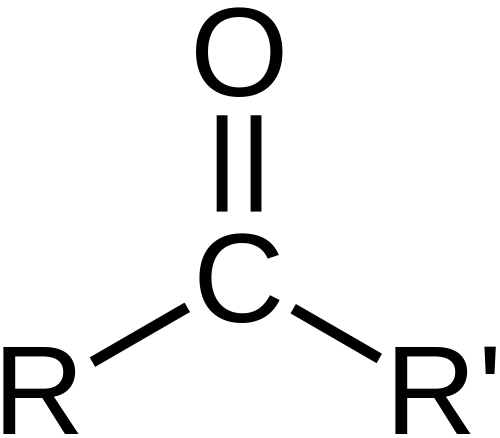
What is Ketone?
Definition:
A ketone is a molecule in which a carbon atom is covalently bonded to an oxygen atom to make a carbonyl group and carbons are bonded to this carbonyl.
Properties:
The ketones are molecules which do have high reactivity and can easily react with other substances. This is due to the very polar nature of the carbonyl group which in turn gives the molecule a partial positive charge. This means that other substances are often attracted to the molecule with which they then readily react. In fact, nucleophilic addition and oxidation and reduction reactions are common.
Formation:
There are several ways that a ketone can be formed. Many alcohols and hydrocarbons can be oxidized to form ketones. Molecules called alkynes can be modified by hydration to form ketone molecules.
Examples and uses:
There are many examples of ketones including acetone, phenylethanone, and propanone. Ketones form the basis for the production of many useful products in industry. For example, they are often used to make various solvents such as acetone that is widely used as a nail polish remover due to its properties as an excellent solvent. Ketones can also be used to strip paints and lacquers and even to make explosives and are often used in the tanning industry.
Occurrence in nature:
Ketone bodies are formed in the human body when fats are metabolized when there is insufficient glucose to supply the energy of the body. In individuals who have poorly controlled diabetes when excessive fats are broken down, these ketones can accumulate and the person thus often has a fruity smelling breath as a result.
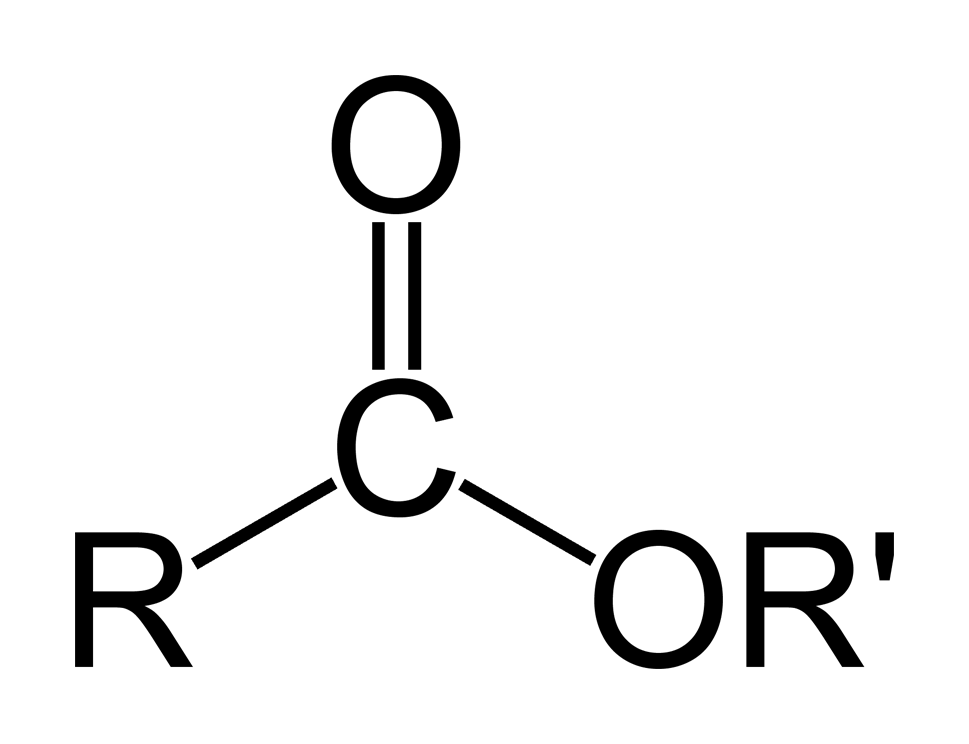
What is Ester?
Definition:
An ester is a molecule which has a carbonyl group and is a molecule in which the hydroxyl has been replaced by a group that is known as an alkoxy (an oxygen atom and an alkyl group).
Properties:
The esters are molecules that have polarity (in other words they have a charge), and therefore do readily react with other molecules. Many small esters are soluble in water but the extent of solubility decreases as the size of the molecule increases. Long chain esters are thus insoluble in water and form part of many lipid molecules. An ester is less acidic than a ketone because of the presence of the alkoxy group. It is also less reactive than is a ketone molecule.
Formation:
Esters can be formed from alcohols, carboxylic acids and certain of the inorganic acids such as nitric acid or phosphoric acid. The process in which carboxylic acid reacts with water in the presence of some type of acid in the presence of heat to form an ester is known as esterification.
Examples and uses:
There are many examples of important esters, such as for instance, acetylcholine and ascorbic acid (vitamin C). Ethyl acetate is an ester that helps give flavor to beer. Some esters are used to make PVC wire insulation since it makes the insulation flexible. Many tricarboxylic acid esters can be found in wine and help to gives wine a specific smell. Esters are used in the manufacture of many substances including Plexiglas and Mylar film. Different esters are used to make dyes, solvents and even gasoline additives.
Occurrence in nature:
Esters of fatty acids make up many of the different fats that occur in nature and are often found in fruits. Pineapples and bananas have esters which give the fruits a specific smell. The waxes of plants are also an example of an ester that is found in nature. Cholesterol esters taken in through the diet are used to form cholesterol in animals, including in humans.
Difference between Ketone and Ester
Definition of Ketone vs. Ester
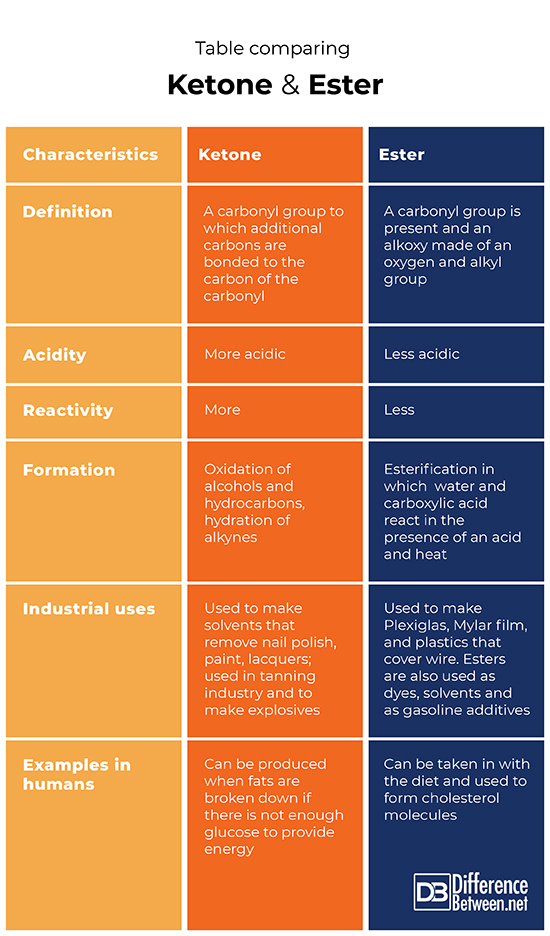
A ketone is a molecule that has a carbonyl bonded to carbons. An ester is a molecule that has a carbonyl and alkoxy group bonded together.
Acidity
A ketone is a more acidic molecule. An ester is a less acidic molecule.
Reactivity
Ketones have more reactivity while esters have less reactivity.
Formation
The oxidation of alcohols and hydrocarbons can produce a ketone as can the hydration of alkynes. The esterification reaction in which carboxylic acid and water are reacted can produce an ester.
Industrial uses
Ketones have many uses including as solvents such as nail polish removers and paint and lacquer removers, and they can be used to produce explosives and in the tanning industry. Esters also have several uses including as solvents, dyes, as additives in gasoline and to make Mylar film and Plexiglas.
Examples in humans
The ketones are formed when fats are broken down if there is not enough glucose in the body to produce energy. Esters can be taken in through the diet and used to form cholesterol in humans.
Table comparing Ketone and Ester
Summary of Ketone Vs. Ester
- Ketones and esters are both molecules that contain a carbonyl group, but there are different atoms attached to the carbonyl in each case.
- Both ketones and esters are molecules that can be used as solvents in certain situations.
- Ketones are more reactive and acidic than esters.
- Esters and ketones both occur in living organisms.
- Difference Between Rumination and Regurgitation - June 13, 2024
- Difference Between Pyelectasis and Hydronephrosis - June 4, 2024
- Difference Between Cellulitis and Erysipelas - June 1, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Farmer, S. et al. “Organic Chemistry”. Libretexts. University of California, 2016, https://chem.libretexts.org/Core/Organic_Chemistry
[1]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/8/83/Ketone-general.svg/500px-Ketone-general.svg.png
[2]Image credit: https://commons.wikimedia.org/wiki/File:Ester.png
