Difference Between Acetone and Bleach
Acetone and bleach are very common household and laboratory chemicals with pungent odors which are typically used for cleansing purposes. Notably, it is dangerous to mix these two compounds as they will form a deadly chemical, chloroform, which could cause serious organ damage. Bleach was first discovered for whitening fabric and acetone was later discovered for the production of British rifle cartridge. Specifically, acetone is an organic compound which is also present in the human body while bleach is a chemical inorganic product with whitening properties.
What is Acetone?
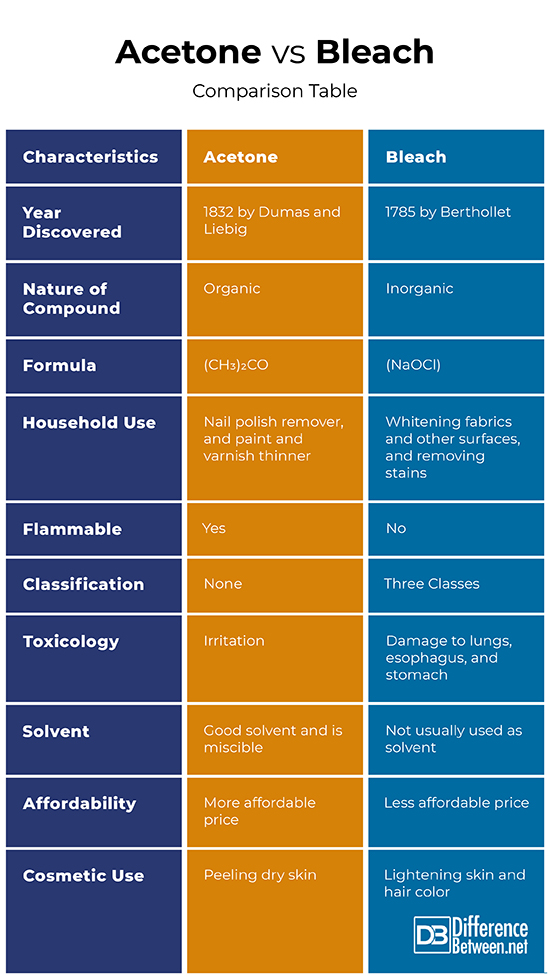
The discovery of acetone is credited to Jean-Baptiste Dumas, a French chemist and Justus von Liebig, a German chemist as they first identified its empirical formula in 1832. The chemical’s first industrial production was during the World War I by Chaim Weizmann, a biochemist. Weizmann’s production process was highly instrumental for the British war commerce.
Acetone is a volatile, flammable, and colorless liquid which is usually utilized in cleaning laboratory equipment. It is an organic compound with the formula (CH3)2CO. Since acetone combines well with water, it is used as a paint thinner and is a main component of nail polish removers. Interestingly, this compound is also found in human blood and urine and that those with diabetes produce higher levels.
What is Bleach?
The advent of chlorine-based bleaches began in 1785 when Claude Berthollet, a French chemist, realized that chlorine could be used in whitening fabrics. The first commercial bleach was also produced by Berthollet when he formulated sodium hypochlorite (NaOCl) and he named it “Eau de Javel” or “Javel Water” which was the town where it was first manufactured. An alternative for Eu de Javel, calcium hypochlorite, was initiated by Charles Tennant, a Scottish chemist in 1798, who also patented bleaching powder a year later. A related milestone in medical practice is attributed to Labarraque, a French chemist, when he proposed the deodorizing and disinfecting utilization of hypochlorites for sanitation in hospitals as well as in industries.
Bleach often refers to a solution of sodium hypochlorite which is used for whitening, lightening, and cleaning purposes. This chemical product is not only for fabrics but also for hair, water, and various kinds of surfaces. Its sanitation effect is also suitable for removing molds and killing weeds. Generally, bleaches are currently classified as chlorine-based, peroxide-based, or sulfur dioxide based.
Difference between Acetone and Bleach?
-
Year Discovered
Acetone’s empirical formula was discovered in 1832 by Dumas and Liebig while chlorine-based bleaches began earlier in 1785 when Berthollet prosed its use for whitening fabrics.
-
Nature
Acetone is an organic compound found in human blood and urine while bleach is an inorganic chemical product.
-
Formula
The formula of acetone is (CH3)2CO while that of chlorine-based bleach, the most common classification is NaOCl.
-
Household Use
Acetone is most often associated with removing nail polish and mixed with paint as a thinner while bleach in various brand names is generally for whitening fabric, removing stains, and lightening the color of differing surfaces.
-
Flammable
Acetone is defined as a flammable liquid while bleach is not flammable on its own. However, bleach can form explosive compounds when combined with acetylene, ammonia, or similar materials.
-
Classification
Bleach has three classes: chlorine-based, peroxide-based, and sulfur dioxide based. On the other hand, acetone has no such classification as it does not have differing active agents.
-
Toxicology
As compared to bleach, acetone poses less danger when ingested as the worst reported case was systemic toxicity with the patent being able to fully recover. It can also cause eye or skin irritation. On the contrary, ingestion of bleaches can lead to esophageal and stomach damage and even possibly worsening to death. Also, one’s lungs can be damaged after inhaling bleach fumes.
-
Solvent
Unlike bleach, acetone is a very useful solvent for certain synthetic materials such as paint, varnish, resin, glue, and other adhesives. Acetone is also used as a denaturant (substance that can chemically change the nature of other substances) and excipient (inactive substance that functions as a medium for other active substances) in pharmaceutical procedures. Also, acetone is miscible which means that it is able to mix in all proportions with other substances.
-
Affordability
As compared to bleaching products, acetone is generally more affordable due to its greater production capacity.
-
Cosmetic Use
Acetone is mainly used in chemical peeling to remove dry skin. It is also ideal for removing skin adhesives from wigs and mustaches. On the other hand, bleach is generally used for lightening skin and hair color.
Acetone vs Bleach: comparison table
Summary of Acetone vs Bleach
- Acetone and bleach are very common household and laboratory chemicals
- Acetone is a volatile, flammable, and colorless liquid.
- Bleach often refers to a solution of sodium hypochlorite which is used for whitening, lightening, and cleaning purposes.
- Acetone was discovered in 1832 while bleach was discovered earlier in 1785.
- Acetone is organic while bleach is inorganic.
- The formula of acetone is (CH3)2CO while that of chlorine-based bleach is (NaOCl).
- Bleach is generally used for whitening fabrics and other surfaces while acetone is used in nail polish removers and paint thinners.
- As compared to acetone, bleach is more toxic. However, acetone is flammable while bleach is not.
- Unlike acetone, bleach has three classifications based on its main ingredient.
- Unlike bleach, acetone is a solvent and is miscible.
- Acetone is more affordable than bleach.
- Regarding cosmetic use, acetone is for peeling dry skin and removing skin adhesives while bleach is for lightening skin and hair color.
- Difference Between Hematoma and Melanoma - February 9, 2023
- Difference Between Bruising and Necrosis - February 8, 2023
- Difference Between Brain Hematoma and Brain Hemorrhage - February 8, 2023
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://pixabay.com/en/bleach-detergent-laundry-147520/
[1]Image credit: https://www.flickr.com/photos/pheezy/83658850
[2]Icon Group International. Acetone. San Diego, CA: Icon Group International, 2008. Print.
[3]Icon Group International. Bleaching. San Diego, CA: Icon Group International, 2008. Print.
[4]Martin, Geoffrey. Chlorine Chlorine Products. London: Forgotten Books, 2017. Print.