Difference Between Sinovac Vaccine and Moderna Vaccine
What are Sinovac vaccine and Moderna vaccine?
Both are COVID-19 vaccines and at present globally being administered to global health workers and other front-end workers who are at an increased risk of virus exposure. However, Moderna vaccine is more efficient in comparison to Sinovac.
Similarity
Similarities between Sinovac vaccine and Moderna vaccine include;
- Administered as COVID-19 vaccines
- Both cause side-effects which are short-lived and minor
Sinovac vaccine
This vaccine is developed by China’s Sinovac Biotech (Beijing-based biopharmaceutical company). The vaccine is known as CoronaVac. As of now, the vaccine is 50.4 percent effective in Brazilian clinical trials. The vaccine has showed 91.25 percent efficacy in Turkey trials and 65.3 percent efficacy in Indonesia trials.
Sinovac vaccine is being used by the general masses of the Republic of China by China’s medical products regulator.
Brazil, Laos, Colombia, Chile and Uruguay have announced to use CoronaVac vaccine by China’s Sinovac company by granting it an emergency authorization. Sinovac vaccine showed some mild adverse side-effects detected in its phase 3 trials. However, the company denied serious complications and reactions reported in Turkey, Indonesia and Brazil.
Moderna Vaccine
The initial results from Moderna’s vaccine were described two months ago by the United States’ leading expert on contagious/infectious diseases Anthony Fauci as “amazingly impressive”.
It has been developed by the US company and has received $2.48 billion in US federal funds. This vaccine is very effective and very safe in individual’s with existing medical complications with an elevated risk of chronic illnesses like blood sugar, hypertension, severe infections, asthma, liver and kidney disease.
The vaccine is not advised and recommended by medical authorities to individuals younger than eighteen years of age due to unavailability of further studies.
The Moderna vaccine has been approved by EMA – European Medicines Agency (EMA) and has also confirmed meeting World Health Organization’s (WHO’s) criteria for SAGE (Strategic Advisory Group of Experts) consideration.
The EMA has thoroughly assessed the data on the quality, safety and efficacy of the Moderna COVID-19 vaccine, due to its ninety four percent efficacy and safety is being used all across the EU – European Union.
People should be careful as it is not advised to individuals who suffer from allergies. So, people who experience severe allergic reaction to the first dosage of the vaccine should not go for the second additional dose.
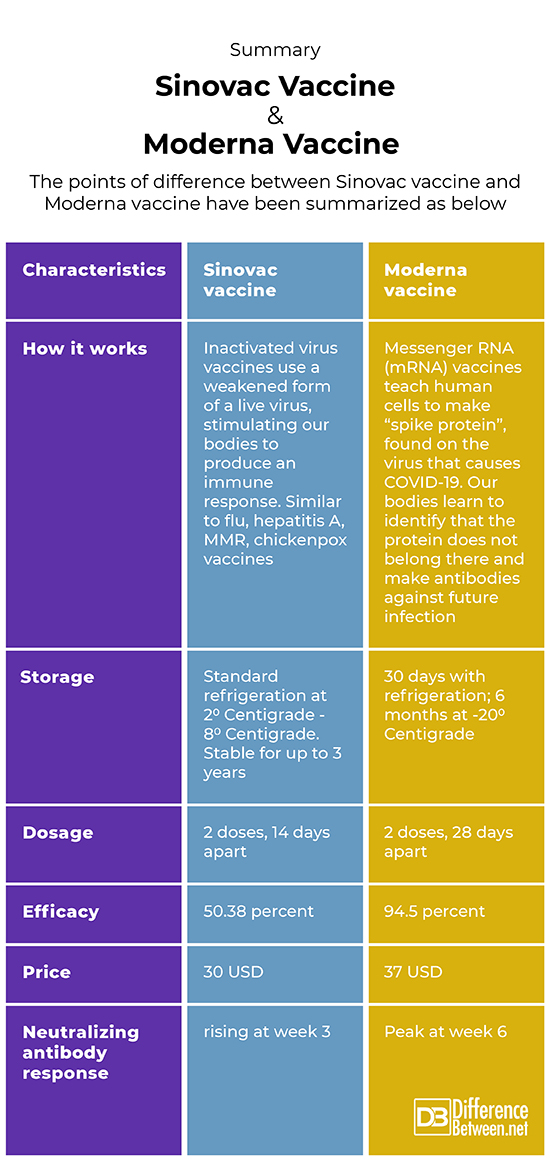
Difference between Sinovac vaccine andModerna Vaccine
Description/How does the vaccine work?
Sinovac vaccine
Sinovac’s vaccine (Beijing-based biopharmaceutical company) makes use of inactivated vaccine technology, which uses a fragile and not so strong form of a live virus to invigorate our bodies to release an immune response. This Sinovac vaccine is similar to the flu and chickenpox vaccines.
Moderna Vaccine
The Moderna vaccine (mRNA-1273) uses mRNA technology.
Cost
Sinovac vaccine
The cost is around 13.6 USD – 30 USD.
Moderna Vaccine
The cost is around 25 USD – 37 USD.
Type
Sinovac vaccine
Inactivated
Moderna Vaccine
mRNA – The Moderna COVID-19 (mRNA-1273) vaccine
Study location
Sinovac vaccine
International (Developed in China)
Moderna Vaccine
United States (Massachusetts)
Side effects
Sinovac vaccine
- Fatigue
- Muscle pain
- Headaches
- Sore arms and fevers are common
- Tenderness and redness near the shot area
Moderna Vaccine
This vaccine has proved to be very effective. However, people have experienced some side-effects especially after the second dose. These side-effects include;
- Tenderness and redness near the shot area
- Fatigue
- Chills
- Nausea and puking
- Pain in the arm
- Lymph nodes ger swollen
Vaccine dosage
Sinovac vaccine
Either 3 μg or 6 μg, at days 0, 7 and 14 (i.e two boosters)
Moderna Vaccine
Either 10 μg or 100 μg, at Day 0 and again at day 28
Neutralizing antibody response
Sinovac vaccine
Could be still rising at week 3
Moderna Vaccine
Peak at week 6, stronger with 100 μg
Storage
Sinovac vaccine
Sinovac vaccine can be stored at normal/standard refrigerator temperatures that is 2 degrees – 8 degrees C and are expected to remain stable for around 3 years. Considering this could be an attractive choice for areas where access to cold temperatures (refrigeration) is difficult and challenging.
Moderna Vaccine
Moderna vaccine can be stored for thirty days with refrigeration, 6 months at -20 degrees C.
Summary
The points of difference between Sinovac vaccine and Moderna vaccine have been summarized as below:
- Difference Between Global Warming and Greenhouse Effect - May 18, 2024
- Difference Between Vaccination and Immunization - March 3, 2024
- Difference Between Selective Mutism and Autism - February 25, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Image credit: https://live.staticflickr.com/65535/50136889878_a7c1a0ea93_b.jpg
[1]Image credit: https://live.staticflickr.com/65535/50770417891_3e8904ab83_b.jpg
[2]Mahase, E. (2020). Covid-19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ: British Medical Journal (Online), 371.
[3]Mahase, E. (2020). Covid-19: Moderna applies for US and EU approval as vaccine trial reports 94.1% efficacy. BMJ: British Medical Journal (Online), 371.
[4]Palacios, R., Patiño, E. G., de Oliveira Piorelli, R., Conde, M. T. R. P., Batista, A. P., Zeng, G., ... & Gast, C. (2020). Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac–PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials, 21(1), 1-3.
[5]Zhang, Y., Zeng, G., Pan, H., Li, C., Hu, Y., Chu, K., ... & Zhu, F. (2020). Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases.
