Difference Between Iron and Ferrous Sulfate
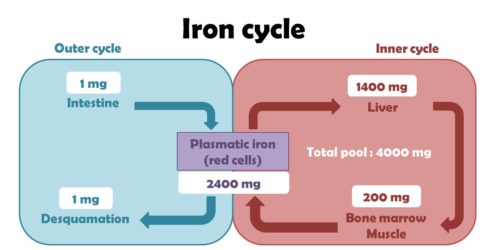
Iron Metabolism in Human Body
At first thought, you may not realize that there is much of a difference between iron and ferrous sulfate. Indeed, most people would assume that the two are the same thing. However, this couldn’t be more false. Whilst both serve the same purposes, they do vary greatly when it comes to how they are created. A closer look at both should provide you with more insight. Shall we get on with it, then?
As you may already know, iron is a trace element found in and necessary to all living organisms. Enzymes and proteins that contain iron are essential in a number of different biological oxidations as well as transport. Within our cells, iron storage is regulated carefully; this is why ‘free’ iron ions are nonexistent. One of the major components required in order for the proper continuation of this process is transferrin, a protein that helps bind iron ions that have been absorbed from the duodenum, and transports these ions through the bloodstream into the cells. In mammals, regulating iron levels is important, as there is great risk for biological toxicity if it is left unattended.
Iron is commonly found in red meat, beans, lentils, fish, poultry, leafy vegetables, chickpeas, tofu, black strap molasses, fortified read, black eyed peas, and fortified breakfast cereals. It is important that people supply their bodies with adequate amounts of iron within their diet so that they do not suffer from iron deficiency. What can you do if you are suffering from the effects of iron deficiency? Well, the intake of iron supplements should help.

Ferrous Sulfate
This is where ferrous sulfate enters the picture. Also known as iron II, ferrous sulfate is a chemical compound often used as a nutritional supplement for people who might be suffering from iron deficiency anemia. However, this is not the only purpose of ferrous sulfate; it is also often used in the manufacturing of inks, including the notable iron gall ink, which was in use during the Middle Ages up until the end of the eighteenth century. Commonly used as dye, ferrous sulfate is also utilized as a mordant in wool dyeing. Harewood, a material typically used in parquetry and marquetry, is made through the use of ferrous sulfate. This chemical compound’s rusty color also makes it suitable for staining limestone, concrete, and even sandstones, in order to create a yellowish tinge.
Horticulturists make use of ferrous sulfate as a treatment for iron chlorosis. It may not be as fast acting as an iron chelate, but the effects are significantly longer lasting. Horticulturists mix ferrous sulfate with the compost and dig the mixture into the soil; this helps them create a store that’s good enough to last them for years on end. Many also utilize the compound as an environmentally friendly moss killer. Moreover, it is also often used as a protective coating for the inside of brass tubes; it is added to the water flowing through the tubes of a turbine condenser, completely coating it with a corrosion-resistant film in the process.
By now, the significant difference is obvious; iron is a chemical element, whilst ferrous sulfate is a chemical compound made up of a number of different minerals. Although they differ so much in structure, they both serve an important purpose when it comes to maintaining a person’s wellbeing. Ferrous sulfate is the form that iron takes in order to be successfully assimilated into our system. You may choose to take it this way – as a supplement – or opt for something more natural – consuming foods rich in iron.
Summary:
- Iron is a chemical element; ferrous sulfate is a chemical compound.
- Iron has to be properly regulated by mammals because of a biological toxicity risk.
- Ferrous sulfate is used to treat iron deficiency anemia.
- Differences Between Fraternity And Sorority - January 8, 2014
- Differences Between Lucite and Plastic - January 7, 2014
- Differences Between Oil and Butter - January 6, 2014
Search DifferenceBetween.net :
19 Comments
Leave a Response
References :
[0]http://2013.igem.org/Team:Evry/Project_metabolism
[1]https://commons.wikimedia.org/wiki/File:Ferrous_sulfate.jpg

I used 65 mg. of iron which was marked equivalent to 325 mg. of ferrous sulfate when the latter was not available. I ended up with sever diarrhea which did not end until I ceased the use of the iron and went looking for the iron sulfate which my doctor had prerscribed.
Iron should be taken on an empty stomach. Didn’t your doctor warned you?
He didn’t say he took it with food. Reactions can happen later on with supplements and meds.
Thanks. I needed to know this. I’d been taking iron and eating iron rich foods, but still couldn’t reach the minimum required (shown in my lab results). The doctor wants me to take ferrous sulfate instead. Interesting about the use in compost mixtures as well.
Thank you for your comment. I wanted to take an organic iron pill and eat iron rich foods but was afraid it wouldn’t work. I’ll just do what the dr prescribed:)
Very helpful comments. I will take the prescribed ferrous sulfate, I thought I’d be able to make up for this by eating more iron rich foods.
Saved me the trouble by reviewing these comments.
Have a great day!
Getting ready to try Ferrous sulfate 325 mg. I am 62. Can i still do my Vit B12 at the same time it’s a supplement. I take it ever other day. not a real meat eater. Plus lost my abbitight. If i spelled that right. I have author all tpyes so i pray this works. It’s nature I love it. Hate Dr. meds don’t trust them. thanks for reading this whoever u are.
Hard to understand how iron can exist in the body without being a compound. Is this unique or what other elements exist in the body without being a compound? Is iron in vivo always chelated? When iron nails are soaked in vinegar for a few days, can our body absorb that iron? How does a doctor choose to prescribe or not prescribe ferrous sulfate versus iron? Elevated iron levels can be harmful, which is why people can benefit from donating blood.
Iron is actually an element and ferrous sulfate is a compound used to treat iron anemia.
I was taking hb fortex didn’t no I wasn’t supposed to take any dairy products with this I was taking it for 3 months when using dairy products even day in tea n take after I guest it might didn’t help at all
My iron levels were 50 points below what they should have been my dr gave me Ferrous sulfate 325mg. When I went to a different dr I explained that I wanted to be back on this b/c it helped with my RLS. This DR gave me Ferrous glucosamine 324mg. I looked on his computer with him and I thought it was what I had taken before. Should I go ahead and take the Ferrous glucosamine or have him write me a new prescription for the Ferrous Sulfate? And why should I take the same one or the new one. Cindy
Stay away from the doctor go to the nutritionist, Health nutritionist are more knowledgeable in this field of work when it comes to health, phytonutrients, multi minerals and vitamins.
I’m taking this. I just LOVE the side effects! If I take it on an empty stomach, I have horrible stomach pains and very nauseous, sometime to the point of puking. If I take it with food I have violent explosive diarrhea. And whether I take it on an empty stomach or with food – no matter what – I feel terrible for a few hours after taking it. I’m talking light headed, shaky, high as a kite, out of body, headache, stomach upset, seeing pink elephants everywhere
The list goes on.
I just want to feel better.
I was taking straight iron tablets, bought iron 65mg/325mg ferrous sulfate by accident. Should I be concerned about the difference? What is the difference? My iron count was good w/iron tablets (per blood tests) will the ferrous sulfate change that?
Where can I find Ferrous Sulfate 325 mg?
Thank you for explaining the difference between Chelated and Sulfate.
My ferritin is on the 3000…my pcp said no iron of any kind. But I take velphoro and my dietitian said iron is not being absorbed. And velphoro helps keep my phosphorus low. Im confuse please give me an answer.
Hi, I’m suffering pre menopausal bleeding i was adviced by a nurse to take ferrous sulfate 65mg tablet..but before that i had already taking multivitamins with iron..so in the morning itake my ferrous and in the evening i take the multivitamims with .can it be harmful for me??
If you have trouble with nausea taking ferrous sulfate, please be sure to buy the slow release kind. I had the same thing but when I switch to the slow release it was a huge difference! I didn’t need to worry if it was taken on an empty stomach or with food.