Difference Between Ultracapacitor and Battery
Energy storage has recently become one of the much talked about topics in the community, ranging from the use of electric or hybrid batteries in the electric vehicles to the chemical forms of electrical storage. But there are still some confusion regarding the distinction between physical and chemical forms of energy storage. In the recent years, lot of resources is being invested towards the study of electrochemical capacitors, the basic functionality of which is storage and supply of electrical energy. Particularly, the high power ultracapacitors have become the center of attraction of the intense research and considered as a potential future of the energy storage devices. The reason ultracapacitors were able to garner so much attention is that they are a great eco-friendly alternative to batteries and can complement batteries for increased backup power.

What is a Ultracapacitor?
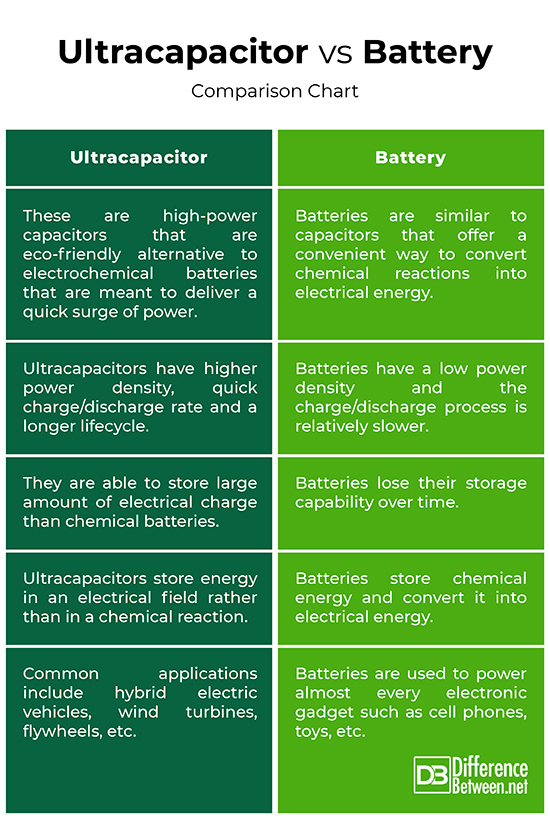
Ultracapacitors, also known as supercapacitors, are a monumental breakthrough in capacitor technology and a source of electrical energy with virtually limitless lifetime. These are double layer capacitors that offer significantly high energy density, meaning they are able to hold hundreds of times more electrical charge compared to standard capacitors. Ultracapacitors are bigger capacitors that are inherently better energy storage devices that are able to store large amount of electrical charge than chemical batteries. Compared to similar size batteries, ultracapacitors have higher power density, quick charge/discharge rate and a longer lifecycle. Ultracapacitors can be used wherever a large amount of power is required for a short period of time. Unlike standard capacitors, ultracapacitors have an electrolyte between the plates rather than a dielectric, meaning they store energy in an electric field rather than in a chemical reaction. Unlike batteries, they are resilient to high temperatures and charge much faster than batteries.

What is a Battery?
Batteries are similar to capacitors that offer a convenient way to convert chemical reactions into electrical energy. The electrical energy can then be used to produce light, heat or motion. Almost every electronic device runs on batteries. For example, TV remote, cell phones, flashlights, toys, or even our vehicles are powered by batteries. The two electrical terminals of a battery are separated by a chemical substance called an electrolyte, which is a medium that allows the flow of electrical charge between the two terminals. When the battery is connected to an external circuit board, the electrolytes move as ions allowing the chemical reactions to be completed at the terminals and thereby delivering energy to the external circuit. It is the movement of the ions within a battery that allows current to flow out of the battery. The term battery was first used in 1749 by Benjamin Franklin wherein he described a battery as a group of capacitors he hooked together to perform some kind of experiments.
Difference between Ultracapacitor and Battery
Basics
– Batteries are a group of two or more electrochemical cells that store are able to store electrical charge and generate current by converting chemical reactions into electrical energy. The electrical energy can then be used to produce light, heat or motion. Almost every electrical or electronic device runs on batteries. Ultracapacitors, on the other hand, are high-power capacitors that are eco-friendly alternative to electrochemical batteries that are meant to deliver a quick surge of power.
Properties
– Ultracapacitors are bigger capacitors that are inherently better energy storage devices that are able to store large amount of electrical charge than chemical batteries. Unlike batteries, they are resilient to high temperatures and charge much faster than batteries. And they are able to store much more energy than standard capacitors. Compared to similar size batteries, ultracapacitors have higher power density, quick charge/discharge rate and a longer lifecycle.
Working
– A battery has two electrical terminals called electrodes which are separated by a chemical substance called an electrolyte which holds electrically charged particles or ions. When the ions combine with the substances that make up the two terminals, a chemical reaction occurs releasing electrical energy. Unlike batteries, ultracapacitors store energy in an electrical field rather than in a chemical reaction and they work electrostatically instead of reversible chemical reactions.
Use
– The two primary uses of ultracapacitors include temporary backup power and supplying peak power. They can be used as a backup power to deliver short-term emergency power when the primary power source is not sufficient enough. Ultracapacitors are a great eco-friendly alternative to batteries; in fact, they are an extension of batteries that increase their lifecycle while reducing the size of the battery. Common applications include hybrid electric vehicles, wind turbines, flywheels, KERS (Kinetic Energy Recovery Systems), and so on.
Ultracapacitor vs. Battery: Comparison Chart
Summary
Energy storage has become increasingly important in recent years, which led to the development of more energy efficient ultracapacitors, which offer an eco-friendly alternative to batteries for energy storage. Ultracapacitors are bigger capacitors that are inherently better energy storage devices that are able to store large amount of electrical charge than electrochemical batteries and they are more resilient to temperatures and charge much faster than batteries. Unlike batteries, ultracapacitors store energy in an electric field. However, they have a limited lifecycle and take much longer to charge.
- Difference Between Caucus and Primary - June 18, 2024
- Difference Between PPO and POS - May 30, 2024
- Difference Between RFID and NFC - May 28, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Deshpande, R.P. Ultracapacitors: Future of Energy Storage. New Delhi, India: McGraw Hill Education India, 2014. Print
[1]Miller, John M. Ultracapacitor Applications. London, United Kingdom: Institution of Engineering and Technology, 2011. Print
[2]Christensen, Victoria G. How Batteries Work. Minnesota, United States: Lerner Publishing Group, 2016. Print
[3]Image credit: https://pixabay.com/da/photos/batteri-alkaline-batterier-opkr%C3%A6ve-4909974/
[4]Image credit: https://commons.wikimedia.org/wiki/File:Skeleton_Technologies_SkelCap.jpg
