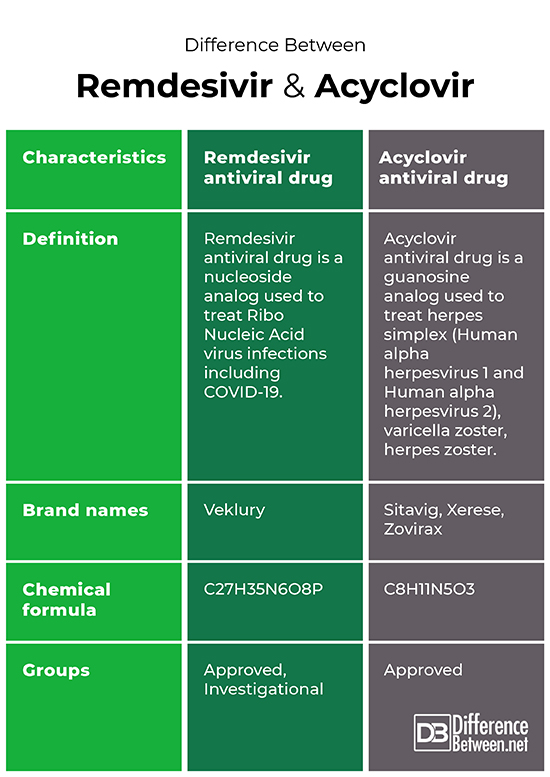
Difference Between Remdesivir and Acyclovir

What is Remdesivir and Acyclovir?
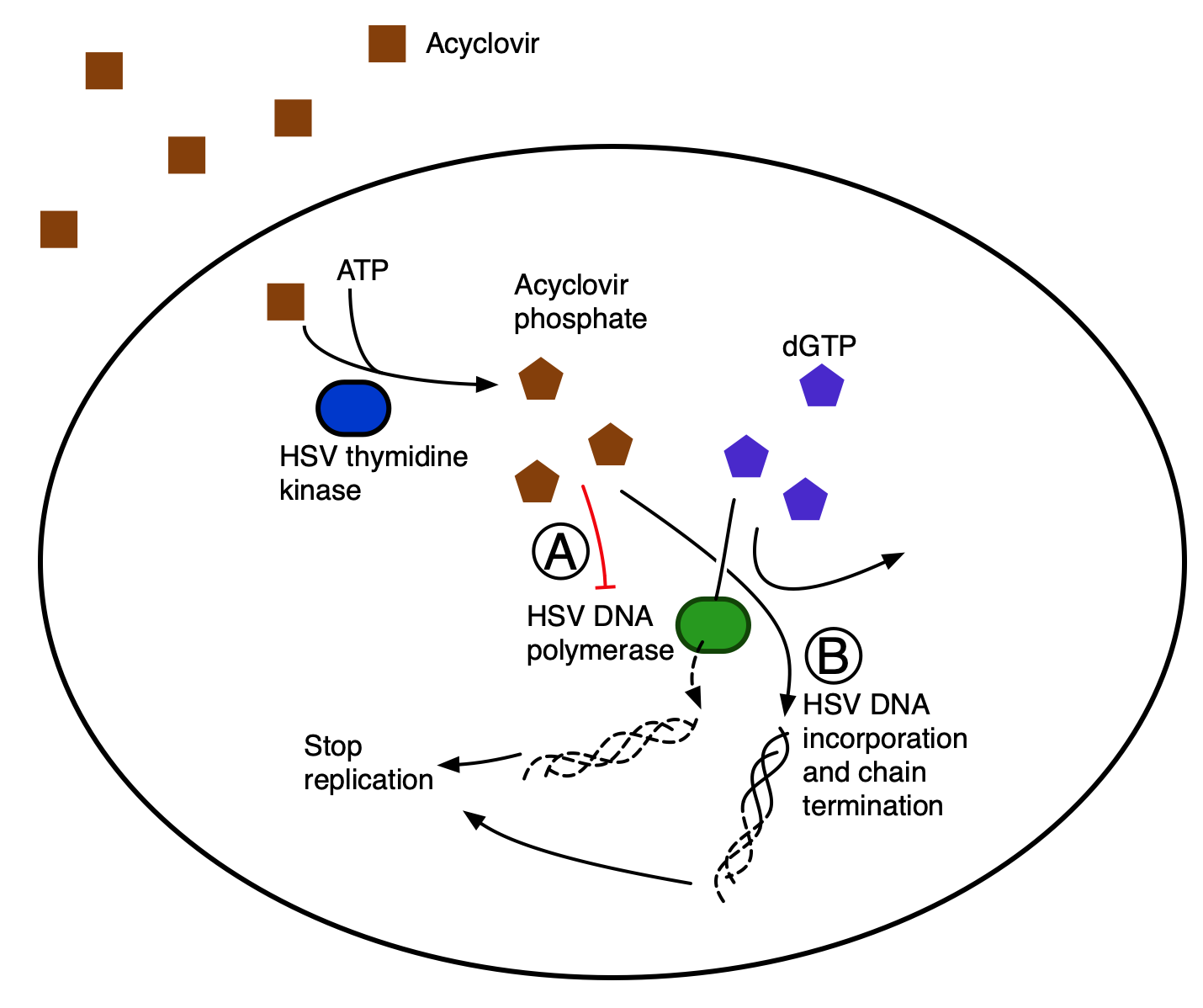
Acyclovir is an antiviral drug used to treat cold sores around the mouth due to herpes, shingles and chickenpox. The drug is also used to treat genital herpes. Acyclovir drug minimizes the episodes in case of recurrent outbreaks. The drug slows down the progression and spread of the herpes virus in the body. The drug is antiviral but it is not a cure for these viral infections. Acyclovir reduces the severity and duration of these outbreaks. Also, people with weak immunity, can use acyclovir to reduce the probability of virus spreading to other parts of the body and resulting in serious infections. However, in March 2020, the drug was declared invalid for treatment of COVID-19 patients. It has been a cheap, safe, and well tolerated alternative for mild cases of coronavirus cases.
Currently, remdesivir is the only FDA – Food and Drug Administration approved antiviral for coronavirus (COVID-19). COVID-19 virus is tricky and is able to proofread or in authenticate the viral RNA genome in case of Acyclovir drug. But in case of Remdesivir drug, the COVID-19 virus ‘s proofreading powers are unable to proofread the viral RNA genome. So remdesivir prevents COVID-19 virus replication and represents a promising, safe and effective drug option for the coronavirus patients.
The antiviral remdesivir minimizes the recovery time for patients hospitalized with coronavirus (COVID-19). Remdesivir drug also shows its effectiveness against Ebola virus RMA and the SARS Coronavirus and MERS (Middle Eastern Respiratory syndrome).
Acyclovir drug could be administered as a potential add-on medication/treatment besides the COVID-19 treatment regimen.
Similarity
Both remdesivir and acyclovir are antiviral drugs
Difference between remdesivir and acyclovir antiviral drugs
Background
Remdesivir
Remdesivir antiviral drug (an adenosine triphosphate) was first described as a treatment for Ebola virus in 2016. It showed promising demonstration in vitro mechanism against Coronaviridae viral families (coronavirus) in 2017 and that lead to a significant interest in this anti-viral drug as a possible treatment for COVID-19. The antiviral drug – Remdesivir had been considered and investigated in several COVID-19 clinical trials.
Remdesivir was granted The United States Food and Drug Administration Emergency Use Authorization (EUA) on 1st May 2020. It was given full approval for treating COVID-19 on 22nd October 2020. Remdesivir in combination with Baricitinib (also known as Olumiant) for the treatment of COVID-19, was granted an The United States Food and Drug Administration Emergency Use Authorization on 19 November 2020.
Acyclovir
Acyclovir is a nucleotide analog antiviral found to be useful for treating herpes simplex (Human alpha herpesvirus 1 and Human alpha herpesvirus 2), Varicella zoster, herpes zoster (Human alphaherpesvirus 3 (HHV-3), shingles and acute genital keratitis. This anti-viral drug is benefitted by patients as it is used as a first line in the treatment of these viruses. Acyclovir was granted The United States Food and Drug Administration approval on 29 March 1982.
Application
Remdesivir
This antiviral drug is used for treatment of Covid-19
Acyclovir
This antiviral drug is used for treatment of Herpes
Protein binding
Remdesivir
Remdesivir is 88-93.6 percent bound to plasma proteins of humans
Acyclovir
Acyclovir antiviral drug is 9-33 percent protein bound in plasma
The points of difference between remdesivir and acyclovir antiviral drugs have been summarized below;
Difference between remdesivir and acyclovir
Why It’s So Hard to Make Antiviral Drugs for COVID?
Viruses are tricky. They keep evolving in mutated forms and strains and destroying a virus is out of question. An anti-viral drug does not destroy a virus but traps it in a cell and prevents it from reproducing.
Basically, antiviral drug development has been lagging in comparison to antibiotic drug development. One anti-viral drug may not be effective for all virus strains. The reason is that since the viruses’ code for only a handful of proteins of their own, a particular anti-viral drug may be able to target only one or two of such proteins. And those viral enzymes (viral protease (PR), the reverse transcriptase (RT), and the integrase (IN)) may have similar functions as performed by the host cells. This imbricate makes the antiviral to negligently damage healthy human cells.
Is Remdesivir authorized in Canada for the treatment of severe COVID-19 conditions?
Remdesivir was legally authorized by medical authorities in Canada on 27th July, 2020. However, some conditions were also put. The drug is meant only for the treatment of patients experiencing severe coronavirus (COVID-19) symptoms like pneumonia needing supplemental oxygen to breathe. The age criteria for patients are – adults aged twelve and over and weighing at least forty kgs. The medicine is strictly prohibited to be administered to children below 12 years of age or pregnant women. Also, the drug is to be administered only intravenously and only in registered health establishments/centers where patients after being administered the drug can be closely monitored. The medical authority to approve the drug (Health Canada) has approved the drug with conditions for the drug manufacturer. The drug manufacturers have to submit to health Canada;
- Reports highlighting any side-effects of this drug which are serious
- Post-market safety monitoring documents or reports
- Any foreign regulatory actions associated with the safety of remdesivir
- Information associated with safety and efficacy of the drug
- Data and information confirming that the drug manufacturing processes and controls will ensure that the drug manufacturing meets all the standards
How long does COVID-19 last on surfaces?
On some surfaces like copper, fabric (cotton), the virus usually dies after a few hours.
Some studies reported that the COVID-19 virus on plastic stays for up to 3 days. However, some Lancet scientists have reported that the coronavirus could be detected on plastic for longer – up to seven days. On paper surface, the virus could be detected for up to 4 days.
How long does it take for acyclovir to work?
After acyclovir is taken orally, it may take up to 2 hours for the response and effectiveness to reach peak plasma concentrations. For herpes symptom reduction, the drug may take up to 3 days to show its effectiveness. Nevertheless, the acyclovir should be taken until the medication course prescribed is completed. Acyclovir when administered orally works best when started within forty-eight hours of symptom onset.
Who should not take acyclovir?
The conditions like reduced kidney function and dehydration are contraindicated with acyclovir drug. It is important to check with your doctor before taking this drug if you have any of these conditions. Women who are pregnant or breastfeeding should also check with their general practitioners before taking acyclovir. Acyclovir should not be given to children who are less than 2 years of age. Anyone having allergy to milk proteins also should not take acyclovir buccal tablets (Sitavig)
What is Acyclovir 400 mg used for?
Acyclovir 400 mg is an anti-viral medication used specifically for Genital Herpes. One should start taking this drug as soon as the symptoms of herpes virus like itching, burning sensation and blisters are experienced. One 400 mg tablet of acyclovir should be taken 3 times a day for 5 days, or even for a longer duration as prescribed by your doctor for recurring herpes.
- Difference Between Global Warming and Greenhouse Effect - May 18, 2024
- Difference Between Vaccination and Immunization - March 3, 2024
- Difference Between Selective Mutism and Autism - February 25, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Hashemian, S. M., Farhadi, T., & Velayati, A. A. (2020). A review on remdesivir: a possible promising agent for the treatment of COVID-19. Drug design, development and therapy, 14, 3215.
[1]Heidary, F., Madani, S., Gharebaghi, R., & Asadi-Amoli, F. (2021). Acyclovir As a Potential Add-On Treatment for COVID-19; A Narrative Review. A Narrative Review (January 17, 2021).
[2]Simonis, A., Theobald, S. J., Fätkenheuer, G., Rybniker, J., & Malin, J. J. (2021). A comparative analysis of remdesivir and other repurposed antivirals against SARS‐CoV‐2. EMBO molecular medicine, 13(1), e13105.
[3]Image credit: https://commons.wikimedia.org/wiki/File:Mechanism_of_acyclovir_action.png
[4]Image credit: https://live.staticflickr.com/65535/50563319461_6318041f7c_b.jpg
