Difference Between Convalescent Plasma Therapy and Hyperimmune Globulin
What is convalescent plasma therapy and hyperimmune globulin?
Administration of convalescent plasma (plasma that is taken from the blood of a patient who has recovered from a disease), serum, or hyperimmune immunoglobulin (H-Ig) is used for treatment of SARIs (severe acute respiratory infections) of viral etiology.
Convalescent plasma therapy is the most reliable treatment for the coronavirus since there are limited options that could be administered quickly.
Hyperimmune globulin (H-Ig) has a longer shelf life and takes longer to develop because of a complex and complicated manufacturing process involved. However, H-Ig offers consistent antibodies in every unit that are virus specific and minimize the risk of getting infected by any kind of virus (not just COVID-19) from donors to patients. Because of the longer shelf life, it is convenient to store and use it in case of future emergency outbreaks. Several clinical trials have proved and given green signal to convalescent plasma therapy to be safe and effective. H-Ig needs to more clinical trials.
Similarity
Both treatments use plasma from recovered patients.
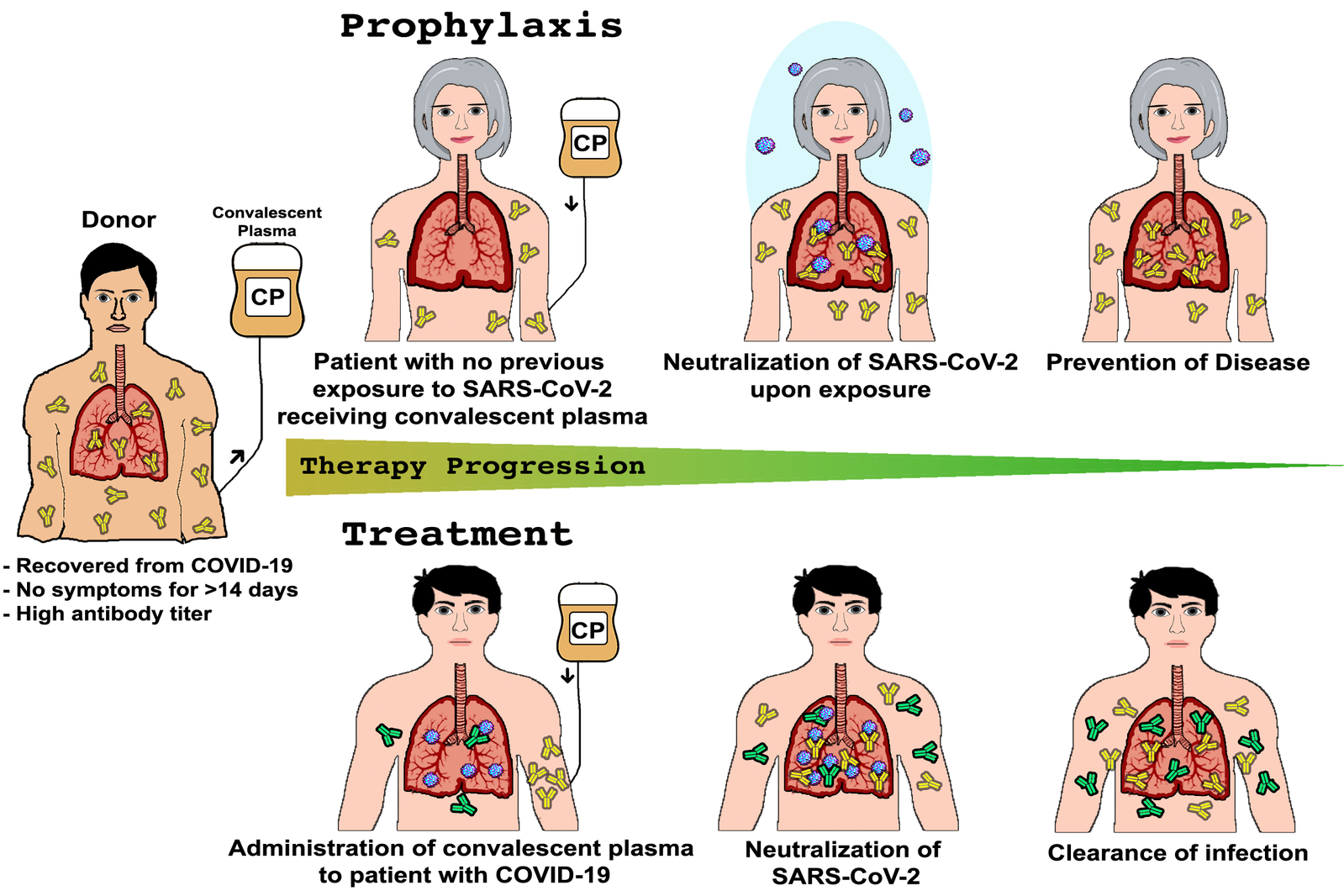
Convalescent Plasma Therapy
Convalescent plasma therapy involves usage of blood form individuals recovered from a sickness/illness to help other sick or ill people recover. The USFDA (The U.S. Food and Drug Administration) gave a green signal to the convalescent plasma therapy for individuals infected with COVID–19 (coronavirus disease). The Food and Drug Administration has recommended use of convalescent plasma therapy during the COVID-19 pandemic since this infection is novel and because of the absence of approved treatment for coronavirus.
Blood from the recovered patients (donors) from coronavirus has antibodies that fight with the virus that causes it. The donated blood from the recovered patients is processed to eliminate blood cells, leaving behind plasma (liquid) and antibodies. These can be given to people with COVID-19 to enhance their immunity to fight the COVID–19 viruses.

Hyperimmune Globulin
Hyperimmune globulins are a class of immunoglobulins (antibodies that play a crucial role in the immune function) for medical use. These immunoglobulins are the preparations that are high in antibodies that safeguard against specific infections and illnesses by providing passive immunity.
The donor (recovered patient) has high titers of antibody (a protein released by the body’s immune system on detection of toxic items known as antigens) against a particular organism or antigen in the plasma.
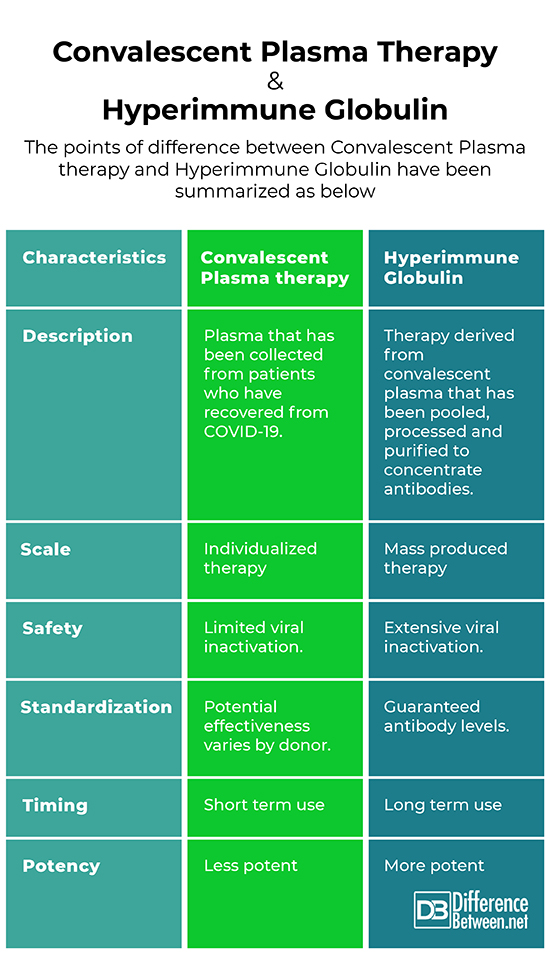
Difference between Convalescent Plasma therapy and hyperimmune globulin
Description
Convalescent Plasma therapy
Plasma that has been collected from patients who have recovered from COVID-19. Transfused directly to people experiencing serious complications from COVID-19
Hyperimmune globulin
Therapy derived from convalescent plasma that has been pooled, processed and purified to concentrate antibodies. A potential global treatment for people at risk for serious complications from COVID-19.
Scale
Convalescent Plasma therapy
Individualized therapy – Plasma form one or several donors is transfused directly to one patient. This approach makes it difficult to scale to the levels needed globally.
Hyperimmune globulin
Mass produced therapy – Plasms from donors who have recovered form COVID-19 is sent to manufacturing facilities. There it is pooled together, processed to remove or inactivate viruses and toxins, and purified to concentrate antibodies.
Safety
Convalescent Plasma therapy
Limited viral inactivation. Scientists must first ensure that donated plasma does not contain other viruses. Other blood characteristics need to be evaluated or tested to ensure compatibility.
Hyperimmune globulin
Extensive viral inactivation. All Hyperimmune Globulin preparations have dedicated viral inactivation or removal steps. Blood type matching is not required.
Timing
Convalescent Plasma therapy
Short term use – Minimal processing means faster availability. Can be available for use the same day plasma is collected, but must be infused or frozen within 24 hours.
Hyperimmune globulin
Long term use – Because it requires more processing and clinical trials, it will take longer for Hyperimmune Globulin to be available. However, it has a longer shelf life (from 24 – 36 months), which makes it easier to distribute and store for use in future outbreaks.
Standardization
Convalescent Plasma therapy
Potential effectiveness varies by donor. Because the amount and range of antibodies in a unit of plasma varies by donor, its potential effectiveness may also vary.
Hyperimmune globulin
Guaranteed antibody levels. Because it is made from pool convalescent plasma that has been purified and concentrated. Hyperimmune Globulin has been standardized so it has a minimum level of antibodies in each unit. The potential effectiveness of various batches should not vary in a therapeutically meaningful way.
Potency
Convalescent Plasma therapy
Less potent because plasma is 90% water and convalescent plasma is minimally processed. It contains fewer virus specific antibodies per unit of volume
Hyperimmune globulin
More potent because it is highly concentrated. Hyperimmune Globulin contains fewer virus specific antibodies per unit of volume
Summary
The points of difference between Convalescent Plasma therapy and Hyperimmune Globulin have been summarized as below:
- Difference Between Global Warming and Greenhouse Effect - May 18, 2024
- Difference Between Vaccination and Immunization - March 3, 2024
- Difference Between Selective Mutism and Autism - February 25, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Chen, L., Xiong, J., Bao, L., & Shi, Y. (2020). Convalescent plasma as a potential therapy for COVID-19. The Lancet Infectious Diseases, 20(4), 398-400.
[1]Cheng, Y., Wong, R., Soo, Y. O. Y., Wong, W. S., Lee, C. K., Ng, M. H. L., ... & Cheng, G. (2005). Use of convalescent plasma therapy in SARS patients in Hong Kong. European Journal of Clinical Microbiology and Infectious Diseases, 24(1), 44-46.
[2]de Alwis, R., Chen, S., Gan, E. S., & Ooi, E. E. (2020). Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine, 102768.
[3]Revello, M. G., Lazzarotto, T., Guerra, B., Spinillo, A., Ferrazzi, E., Kustermann, A., ... & Gerna, G. (2014). A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. New England Journal of Medicine, 370(14), 1316-1326.
[4]Image credit: https://www.zdnet.com/a/hub/i/r/2020/04/21/933f1eb2-653d-42b5-879c-955e73e362fd/resize/1200x900/93d4bd88ee9161000e8b7c5da8d9f5fe/virusistock-1204793213.jpg
[5]Image credit: https://commons.wikimedia.org/wiki/File:Ppat.1008735.g002.png
