Difference Between Supernatant and Precipitate
Supernatant is the name used for the liquid that forms above solid material in a solution. Precipitate is the name used to describe the solid material that forms in a solution.

What is Supernatant?
Definition:
Supernatant is the term used to describe the liquid that forms over some type of solid material. The liquid may or may not be clear depending on what type of components are present in the solution.
Formation process:
Centrifugation often results in supernatant forming because the spinning of the substance causes the various components found in a solution to separate out based on size and density of particles. Particles that are light and very small will separate out near the top while heavier particles will remain near the bottom of a centrifuge tube. It makes sense then that the liquid particles will lie above solid substances. Supernatant may also remain after substances have crystallized.
Separation process:
Supernatant may be separated from solution by pouring the fluid out of the solution and leaving the solid material behind. A pipette can also be used to extract supernatant from a solution once separated from the solid substances.
Uses/applications:
Different types of supernatants have medical and scientific applications. For example, plasma can be separated from red blood cells and used in blood transfusions where only plasma is needed. The supernatant of urine can be used to check for certain kidney diseases and problems.
Examples:
There are several types of supernatants that can be formed as a result of various chemical and physical methods. Examples of supernatant include the plasma of blood and the clear component of urine.

What is Precipitate?
Definition:
A precipitate can be defined as a solid substance that has been produced in a solution.
Formation process:
Centrifuging solutions is a common method of collecting a supernatant and a precipitate. The precipitate forms at the bottom of the centrifuge tube because these molecules are heavier and larger than those found in the liquid above. Another way substances precipitate out is through chemical reactions. In fact, DNA precipitate is formed by adding ethanol to a biological solution containing a mixture of substances.
Separation process:
Similar to the case with supernatant, precipitate is separated by pouring off the liquid component to leave the solid material behind. Another way to separate the precipitate from other substances is through filtration methods.
Uses/applications:
Precipitates are useful in scientific research but also in other fields like waste water treatment. For instance, DNA pellets can be extracted from solution and used for molecular studies in which DNA is sequenced. Precipitation of dangerous metals is done by precipitation methods in wastewater treatment plants. For example, arsenic can be removed through precipitation methods.
Examples:
Sodium chloride can precipitate out in water when the solution has more of the salt than water. DNA and other cellular components can precipitate out after centrifugation.
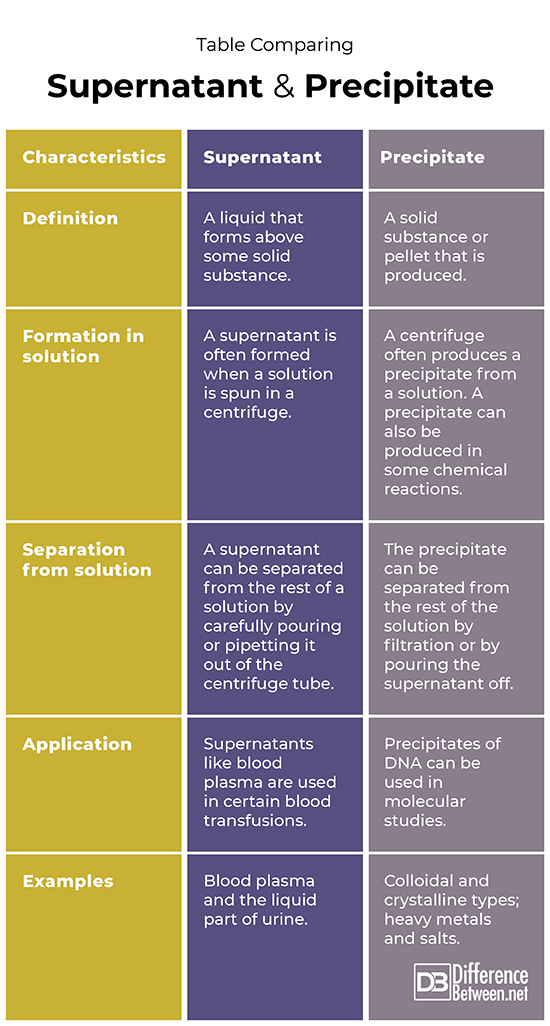
Difference between Supernatant and Precipitate
Definition
A supernatant is the liquid portion of a solution that is formed above solid material. A precipitate is the solid component that occurs beneath the liquid part of a solution.
Formation in solution
A supernatant is often formed when a solution is spun in a centrifuge. A centrifuge often produces a precipitate from a solution and a precipitate can also be produced in some chemical reactions.
Separation from solution
To separate supernatant from the solid material ,one can pour or pipette the liquid out after centrifuging. To separate precipitate from the liquid material you can either pour out the liquid or use some type of filter to separate solid from liquid parts.
Application
Blood plasma is the liquid of blood which is the supernatant left after centrifugation of whole blood; the plasma can be used for blood transfusions. Precipitates of DNA can be used in studies of molecular biology.
Examples
Examples of supernatants include the liquid of urine and blood plasma. Examples of precipitates include DNA and cells in biology, and heavy metals in the case of wastewater treatment.
Table comparing Supernatant and Precipitate
Summary of Supernatant and Precipitate
- Supernatant and precipitate are both components found in solutions.
- The supernatant is the liquid part of a solution that overlies solid material.
- The precipitate is the solid portion of a solution.
FAQ
How do you separate a precipitate from supernatant?
You can either remove the supernatant via pouring it out of the container, leaving the solid precipitate behind, or using a filter that only allows the liquid to pass through it.
What is the difference between supernatant and sediment?
A supernatant is a fluid substance found above a solid substance in a solution. A sediment is solid material that will settle to the bottom of a solution due to having a higher density.
What is the difference between supernatant and supernate?
The term supernate is a noun that is used as a name for the fluid, the supernatant found above the precipitate in a solution after some process in which the liquid and solid have been separated.
- Difference Between Rumination and Regurgitation - June 13, 2024
- Difference Between Pyelectasis and Hydronephrosis - June 4, 2024
- Difference Between Cellulitis and Erysipelas - June 1, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Fukuda, Akihiro, et al. "Excretion Patterns of Urinary Sediment and Supernatant Podocyte Biomarkers in Patients with CKD." Kidney360 3.1 (2022): 63.
[1]Harper, Thomas R., and Neville W. Kingham. "Removal of arsenic from wastewater using chemical precipitation methods." Water Environment Research 64.3 (1992): 200-203.
[2]Olive, Peggy L. "DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells." Environmental and molecular mutagenesis 11.4 (1988): 487-495.
