Difference Between Anhydrous and Hydrous
Organic and inorganic substances may or may not contain water. Based on the presence or absence of water molecules they are divided into hydrous substances (hydrates), which contain water molecules, and anhydrous substances (anhydrates), which do not contain water molecules. A hydrate may be converted to an anhydrate by removing the water molecules from it, and an anhydrate may be converted to a hydrate by adsorbing moisture.

What is Anhydrous?
Anhydrous is a substance that does not contain water molecules in its structure. A hydrate may be converted to an anhydrate by removing the water molecules from it by heating, or by a chemical reaction. The anhydrates are usually highly soluble in water or have big water absorption capacity. Anhydrous compounds have to be stored very carefully, as they gradually tend to absorb moisture from the surrounding air.
Due to their ability to remove the water the anhydrous substances are used in drying agents to absorb moisture and keep different products dry. For example, silica gel is widely used to prevent shoes, bags, toys, etc. from moist. Other anhydrates are used to absorb moisture in tobacco products, food products, etc. and thus prevent the development of molds.
Anhydrous substances are used in chemical reactions, which would be changed by the presence of water. For example, the Grignard reaction, important for the formation of carbon–carbon bonds should be done in the absolute absence of water. If water molecules are available during the reaction they will protonate and thus destroy the highly nucleophilic Grignard reagent, which will change the outcome of the process.

What is Hydrous?
Hydrous is a substance that contains water molecules in its structure. An anhydrate may be converted to a hydrate in the presence of water.
The water molecule is attached to the hydrous substances via a chemical bond with the cation of the molecule. It is known as water of crystallization or water of hydration. Hydrous substances, in general, are stable at room temperature. The so-called efflorescent hydrates can exude water in the atmosphere.
Hydrous substances are widely used for different purposes. For example, gypsum is commonly used in the production of cement, plaster of Paris, wallboard, etc. Hydrates are often used for hydration of the skin in different skincare products. Epsom salt is used to ease muscle soreness and stress and as a natural exfoliant. The naturally occurring mineral borax is used in household cleaning products, as a buffer in chemical laboratories, in various cosmetic products, as a component of glass and ceramics, etc.
Difference Between Anhydrous and Hydrous
Definition
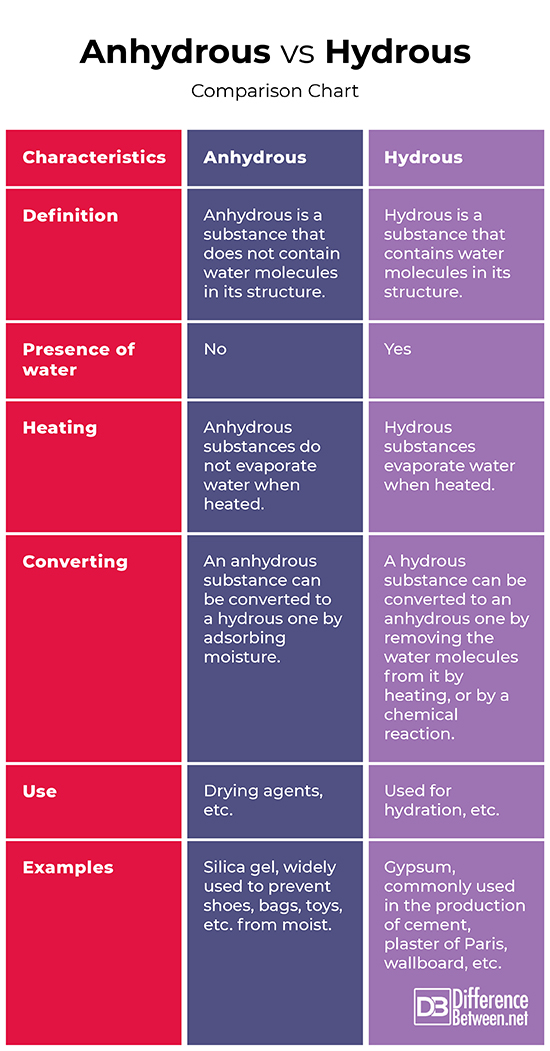
Anhydrous: Anhydrous is a substance that does not contain water molecules in its structure.
Hydrous: Hydrous is a substance that contains water molecules in its structure.
Presence of water
Anhydrous: Anhydrous substances do not contain water.
Hydrous: Hydrous substances contain water of crystallization in their molecules, attached to the cation with a chemical bond.
Heating
Anhydrous: Anhydrous substances do not evaporate water when heated.
Hydrous: Hydrous substances evaporate water when heated.
Converting
Anhydrous: An anhydrous substance can be converted to a hydrous one by adsorbing moisture.
Hydrous: A hydrous substances can be converted to an anhydrous one by removing the water molecules from it by heating, or by a chemical reaction.
Use
Anhydrous: In general, anhydrous substances can be used as drying agents. They are also used for other purposes, depending on the specifics of the substance.
Hydrous: Hydrous substances are widely used for different purposes, depending on the specifics of the substance.
Examples
Anhydrous: Silica gel, widely used to prevent shoes, bags, toys, etc. from moist.
Hydrous: Gypsum, commonly used in the production of cement, plaster of Paris, wallboard, etc.
Anhydrous Vs Hydrous: Comparison Chart
Summary:
- Organic and inorganic substances may or may not contain water.
- Based on the presence or absence of water molecules substances are divided into hydrous and anhydrous ones.
- Anhydrous is a substance that does not contain water molecules in its structure.
- Hydrous is a substance that contains water molecules in its structure.
- A hydrous substance may be converted to an anhydrous one by removing the water molecules, and an anhydrous substance may be converted to a hydrous one by adsorbing moisture.
- Anhydrous substances do not contain water. Hydrous substances contain water of crystallization in their molecules, attached to the cation with a chemical bond.
- Anhydrous substances do not evaporate water when heated. Hydrous substances evaporate water when heated.
- In general, anhydrous substances can be used as drying agents. They are also used for other purposes, depending on the specifics of the substance. Hydrous substances are widely used for different purposes, depending on the specifics of the substance.
- An example of an anhydrous substance is silica gel, widely used to prevent shoes, bags, toys, etc. from moist. An example of a hydrous substance is gypsum, commonly used in the production of cement, plaster of Paris, wallboard, etc.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
Leave a Response
References :
[0]De, A. A Text Book of Inorganic Chemistry. New Delhi: New Age Publishers. 2003. Print.
[1]Housecroft, C., A. Sharpe. Inorganic Chemistry. London: Pearson Education Limited. 2012. Print.
[2]Lazarov, D. Inorganic Chemistry. Sofia: St. Kliment Ohridski. 1995. Print.
[3]Image credit: https://commons.wikimedia.org/wiki/File:V2O5_hydrous.jpg
[4]Image credit: https://commons.wikimedia.org/wiki/File:Anhydrous_cobalt_chloride.jpg
