Difference Between Temperature and Thermal Energy
What is Temperature?
Temperature is a physical property, characterizing the average kinetic energy of the particles of a macroscopic system in thermodynamic equilibrium. It is a property of the matter, which quantifies the concepts of warm and cold. Warmer bodies have a higher temperature than the cooler ones.
The temperature plays an important role in all areas of the natural sciences – physics, geology, chemistry, atmospheric sciences, and biology. Many of the physical properties of the substances, including the solid, liquid, gaseous, or plasma phase, density, solubility, vapor pressure, and electrical conductivity, depend on temperature. The temperature also plays an important role in the determination of the speed and scope of chemical reactions.
Quantitatively the temperature is measured with thermometers. Three temperature scales are currently used in science and industry. Two of them are on the SI system – the Celsius and the Kelvin scales. The Fahrenheit scale is mainly used in the United States.
When two bodies with different temperatures come in a contact, heat exchange takes place between them, causing the warmer body to cool down and the cooler body to heat up. The heat exchange stops when the bodies become with equal temperature. Then thermal equilibrium is established between them.
Temperature is a measure of the intensity of the heat movement of the particles. Brown’s movement becomes more intense when the temperature rises. Diffusion also occurs faster at higher temperatures. These examples show that the temperature is directly related to the chaotic motion of the structural elements. The particles of the heated bodies have higher kinetic energy – they move more intensely. In contact, the particles of the body with higher temperature yield some of their kinetic energy to the particles of the cooler body. This process continues until the intensity of the particles movement in the two bodies becomes equal. Heat phenomena are therefore associated with the chaotic movement of the structural elements, which is why this movement is called thermal.
Due to the chaotic nature of the thermal movement, the particles have a variety of kinetic energies. As the temperature increases, the number of particles that have greater kinetic energy increases, i.e., the heat movement becomes more intense.
When the temperature decreases the intensity of the thermal movement decreases. The temperature at which the thermal movement of the particles is terminated is called absolute zero. The absolute zero on the Celsius scale corresponds to a temperature of -273.16° C.
What is Thermal Energy?
Energy is a physical property that characterizes the ability of a system to change the state of the environment or to execute work. It can be attributed to any particle, object, or system. There are different forms of energy, which often bear the name of the respective force.
The total kinetic energy of the structural elements of a system (atoms, molecules, charged particles) is called thermal energy. It is a form of energy associated with the movement of the structural elements that make up the system.
As the temperature of a body increases, the kinetic energy of the structural elements increases. As the kinetic energy increases, the body’s thermal energy increases. Therefore, the thermal energy of the bodies increases with the increase of their temperature.
Thermal energy depends on the body mass. Let’s take, for example, a cup of water and a lake with the same temperature. At the same water temperature, the average kinetic energy of the molecules is the same. But in the lake the quantity of the molecules and, respectively, the thermal energy of the water are significantly larger.
Transfer of thermal energy occurs whenever a temperature gradient exists in a system of continuous matter. Thermal energy can be transferred by conduction, convection, and radiation. It is transmitted from the parts of a body (or system) with a higher temperature to the parts where the temperature is lower. The process continues until the temperature in the body (or system) equals.
Thermal energy is actually the kinetic energy of the structural elements of the matter. Thermal conductivity is, respectively, a transfer of this kinetic energy and occurs in the chaotic collisions of particles.
Depending on their ability to allow easy movement of the thermal energy the substances are divided into conductors and insulators. The conductors (e.g. metals) allow easy movement of the thermal energy through them, while the insulators (e.g. plastic) don’t allow it.
Nearly every energy transfer is related to the release of thermal energy.
The unit of measurement of thermal energy on the SI system is Joule (J). Another often used unit is Calorie. Thermal energy corresponding to energy at a temperature of 1 K is 1,380 × 10-23 J.
Difference Between Temperature and Thermal Energy
-
Definition
Temperature: The average kinetic energy of the structural elements of a system (atoms, molecules, charged particles) is called temperature.
Thermal energy: The total kinetic energy of the structural elements of a system is called thermal energy.
-
Values
Temperature: The temperature can be positive and negative.
Thermal energy: The thermal energy always has positive values.
-
Units of measurement
Temperature: The temperature is measured in Celsius, Kelvin, and Fahrenheit.
Thermal energy: The thermal energy is measured in Joule and Calorie.
-
Quantitative dependence
Temperature: The temperature does not depend on the quantity of the substance – it is related to the average kinetic energy of the particles.
Thermal energy: The thermal energy depends on the quantity of the substance – it is related to the total kinetic energy of the particles.
Temperature vs Thermal Energy: Comparison Chart
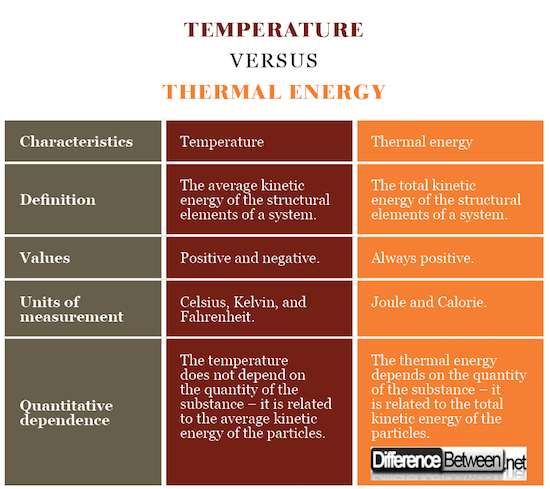
Summary of Temperature vs Thermal Energy
- The average kinetic energy of the structural elements of a system (atoms, molecules, charged particles) is called temperature.
- The total kinetic energy of the structural elements of a system is called thermal energy.
- The temperature can be positive or negative, while the thermal energy always has positive values.
- The temperature is measured in Celsius, Kelvin, and Fahrenheit. The thermal energy is measured in Joule and Calorie.
- The temperature does not depend on the quantity of the substance – it is related to the average kinetic energy of the particles.
- The thermal energy depends on the quantity of the substance – it is related to the total kinetic energy of the particles.
- Difference Between Gallstones and Cholecystitis - September 5, 2021
- Difference Between Constipation and Cramping - August 4, 2021
- Difference Between Whole Genome Sequencing and Microarray - May 6, 2021
Search DifferenceBetween.net :
1 Comment
Leave a Response
References :
[0]Halliday, D. Resnick. Fundamentals of Physics. Textbook. Hoboken: Wiley. 2004. Print.
[1]Kuchling, H. Physics. Moscow: Mir. 1982. Print.
[2]Popov, V. Termodynamics and Statistical Physics. Sofia: University Press: St. Kliment Ohridski. 2009. Print.
[3]Image credit: https://upload.wikimedia.org/wikipedia/commons/thumb/f/f4/Instrumental_Temperature_Record.png/616px-Instrumental_Temperature_Record.png
[4]Image credit: https://upload.wikimedia.org/wikipedia/commons/0/08/NASA_Earth_radiation_thermal_balance_energy_budget_atmosphere.jpg

Dear Dr. Bozhilova, is it possible to calculate how much compost heat is required to capture 1MW of Thermal Energy?
By how much compost I mean how many tons of compost?
Thank you