Difference Between Rapid and PCR COVID-19 Test
We were not prepared then, when a virus of unknown origin first appeared in Wuhan, China and in less than two months, grappled over 150 nations, infecting hundreds of thousands of people worldwide and spreading panic and chaos everywhere. In March 2020, the WHO declared the outbreak a global pandemic and named the novel coronavirus “COVID-19” virus, which has been wreaking havoc since then. Yes, we were not prepared back then, but we are prepared now. In the early stage of the epidemic, the clinical treatment options and the diagnosis of the COVID-19 was rapidly changing and confusing. By now, the medical and science fraternity have understood that the accumulation of data, the understanding of COVID-19, and the importance of reliable testing to contain the spread of the virus have become increasingly apparent. When it comes to COVID-19 diagnosis and detection, the testing can be broadly divided into two categories: Rapid Antigen Test and PCR Test.

What is Rapid Antigen Test?
Rapid Antigen test, commonly referred to as rapid test, is a type of diagnostic test that detects the presence of protein segments, or the antigens for the SARS-CoV-2 virus. Commonly used for diagnosis of respiratory illnesses, rapid test looks for the proteins specific to the SARS-CoV-2 virus, the virus that causes the novel coronavirus disease. Rapid antigen tests are portable diagnostic tools that can be directly operated by physicians or even patients themselves. Recent advances in clinical virology such as the rapid antigen test have made it easy to diagnose infectious diseases, such as COVID-19, in clinical settings with a lesser turnaround time. Antigen assays are relatively economical than molecular techniques but often less sensitive. It is used to determine whether an individual has an active or prior COVID-19 infection based on nasopharyngeal swab specimens. The test is done to identify specific proteins on the surface of the virus, but because of its low sensitivity, if often yield false negative results.

What is PCR Test?
Polymerase Chain Reaction (PCR) is a relatively new approach to detecting novel pathogens directly from clinical specimens. PCR is the gold standard diagnostic method for the testing and detection of SARS-CoV-2 virus. It is an in-vitro technique for the amplification of DNA using a lab technique called PCR. It is a simplified version of the DNA replication process that occurs during cell division. The test looks for the presence of viral RNA i.e. the genetic material of the virus. It uses gene amplification to detect the presence of nucleic acid from individual infectious agents, and the technology is able to detect multiple pathogens from a single patient specimen. A fluid sample is taken from the patient’s nasal swab and the results may be available in minutes depending on various factors. The PCR test promises high specificity, sensitivity and early detection of the COVID-19 infection, which effectively narrows down the viremic window period.
Difference between Rapid and PCR COVID-19 Test
Methods used on Rapid and PCR COVID-19 Test
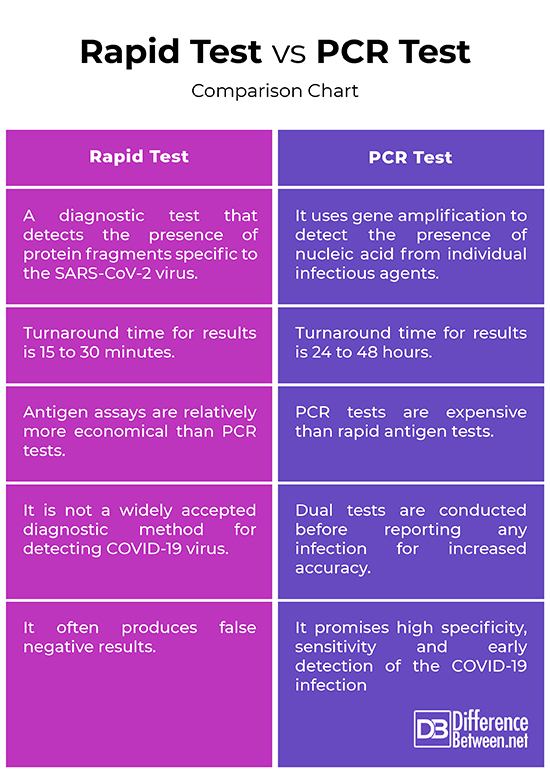
– Rapid Antigen test, commonly referred to as rapid test, is a type of diagnostic test that detects the presence of protein fragments specific to the SARS-CoV-2 virus – the virus responsible for causing the COVID-19 infection. . The test is done to identify specific proteins on the surface of the virus. Polymerase Chain Reaction (PCR), on the other hand, is a relatively new technique used for the detection of novel pathogens directly from clinical specimens. It is a gold standard diagnostic method for testing of COVID-19 that uses gene amplification to detect the presence of nucleic acid from individual infectious agents.
Turnaround Time for both Rapid and PCR COVID-19 Test
– The antigen test is referred to as rapid test is used to determine whether an individual has an active or prior COVID-19 infection based on nasopharyngeal swab specimens. Turnaround time for results is very quick in many cases, meaning you can get the results in as early as 15 minutes, or in extreme cases, it may take one or two days at most. The PCR test looks for the presence of viral RNA i.e. the genetic material of the virus, which gives a clear picture of whether the patient is infected with the virus, and the results may take 24 to 48 hours depending on the laboratory location.
Specificity for Rapid and PCR COVID-19 Test
– Antigen assays are relatively economical than molecular techniques but often less sensitive. Because of its low sensitivity, the rapid test often yields false negative results. The PCR test promises high specificity, sensitivity and early detection of the COVID-19 infection, which effectively narrows down the viremic window period. PCR test is one of the fastest and most accurate laboratory diagnostic methods for the detection of COVID-19 virus, and a positive test result is considered highly confirmatory as dual tests are conducted before reporting any infection.
Rapid Test vs. PCR Test: Comparison Chart
Summary
If you have symptoms similar to that of COVID-19 infection, then PCR test is the ideal test for diagnosis and early detection as it promises high specificity and sensitivity, which effectively helps narrow down the viremic window period. In fact, PCR test is the gold standard for the diagnosis and early detection of the COVID-19 virus. The rapid antigen tests are relatively economical than molecular techniques but often less sensitive and yield false negative results. While the rapid test looks for the presence of protein fragments specific to the SARS-CoV-2 virus, the PCR test detects the genetic material of the SARS-CoV-2 virus.
- Difference Between Caucus and Primary - June 18, 2024
- Difference Between PPO and POS - May 30, 2024
- Difference Between RFID and NFC - May 28, 2024
Search DifferenceBetween.net :
Leave a Response
References :
[0]Lo, Dennis. Clinical Applications of PCR. New Jersey, United States: Humana Press, Inc., 1998. Print
[1]Qu, Jie-Ming, et al. COVID-19: The Essentials of Prevention and Treatment. Amsterdam, Netherlands: Elsevier, 2020. Print
[2]Horton, Richard. The COVID-19 Catastrophe: What's Gone Wrong and How to Stop It Happening Again. New Jersey, United States: John Wiley & Sons, 2020. Print
[3]Hodinka, Richard L., et al. Clinical Virology Manual. New Jersey, United States: John Wiley & Sons, 2020. Print
[4]Anfossi, Laura. Rapid Test: Advances in Design, Format and Diagnostic Applications. London, United Kingdom: IntechOpen, 2018. Print
[5]Adams, David P. Foundations of Infectious Disease: A Public Health Perspective: A Public Health Perspective. Massachusetts, United States: Jones & Bartlett Learning, 2020. Print
[6]Image credit: https://live.staticflickr.com/65535/50688135107_4820af001b_b.jpg
[7]Image credit: https://live.staticflickr.com/65535/49868941148_a126137bb9_b.jpg
