Difference Between Remdesivir and Tamiflu
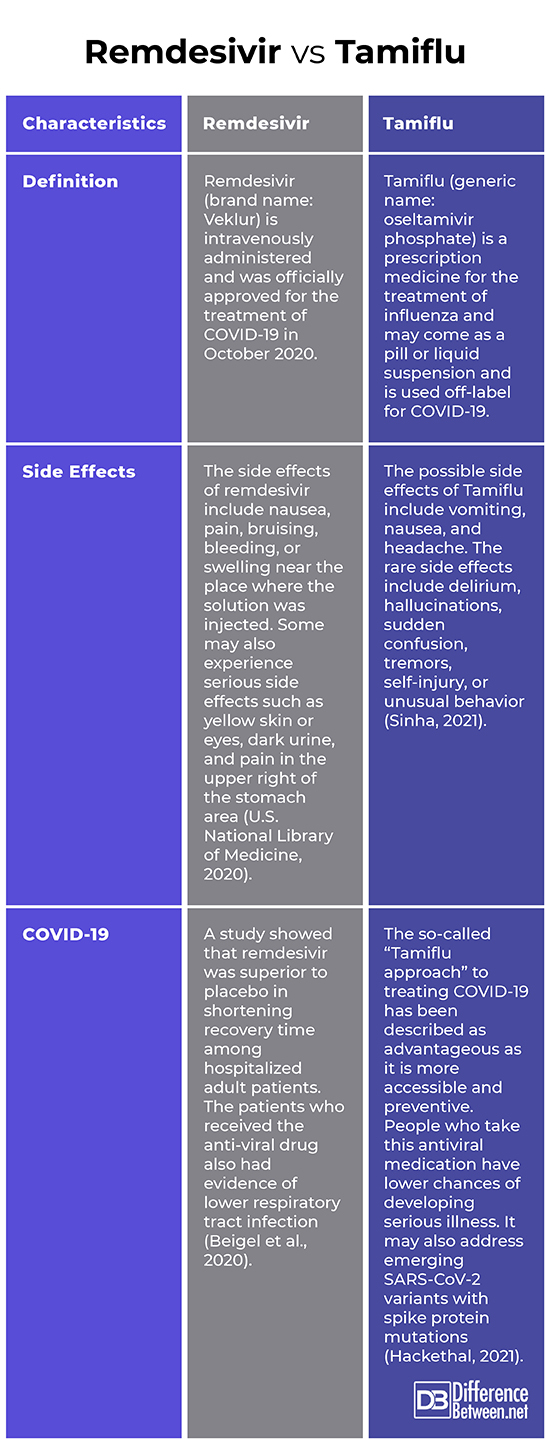
Both remdesivir and Tamiflu are antiviral drugs and have been associated with the treatment of COVID-19. Specifically, remdesivir (brand name: Veklur) is intravenously administered and was officially approved for the treatment of COVID-19 in October 2020. On the other hand, Tamiflu (generic name: oseltamivir phosphate) may come as a pill or liquid suspension and is used off-label for COVID-19. The following discussions further delve into their differences.

What is Remdesivir?
Remdesivir is an intravenously administered antiviral drug. It is described as a “nucleoside analog” which means that it imitates a viral RNA component that the virus needs to replicate itself. It is like it is disguising itself as a building block of viral genetic material (Bai, 2020). Basically, it stops the virus from spreading within the body by preventing it from producing a particular enzyme which is necessary for replication. Initially, remdesivir was created as a possible treatment of hepatitis; it was then later studied as a potential treatment for the Ebola virus. Succeeding research proved that it was effective against Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). However, the trials were only on animals and in test tubes (Hackensack Meridian Health, 2020).
Regarding COVID-19, a study showed that remdesivir was superior to placebo in shortening recovery time among hospitalized adult patients. The patients who received the anti-viral drug also had evidence of lower respiratory tract infection (Beigel et al., 2020). The U.S. Food and Drug Administration (FDA) officially approved remdesivir for the treatment of COVID-19 in October 2020. The drug is given intravenously to hospitalized patients, typically once a day for five to ten days (Hackensack Meridian Health, 2020).
Remdesivir is sold under the brand name Veklur. It comes as a solution (powder to be mixed with liquid) and is slowly injected over 30 to 120 minutes. The side effects include nausea, pain, bruising, bleeding, or swelling near the place where the solution was injected. Some may also experience serious side effects such as yellow skin or eyes, dark urine, and pain in the upper right of the stomach area (U.S. National Library of Medicine, 2020).
What is Tamiflu?
Tamiflu (generic name: oseltamivir phosphate) is a prescription medicine for the treatment of influenza. It specifically blocks the actions of influenza virus type A and type B; it is given to people who are at least 2 weeks of age who have had flu symptoms for no more than two days. Tamiflu is one of the four FDA-approved antiviral drugs which was recommended by the Centers for Disease Control and Prevention (CDC) to treat flu. The other three are Relenza (generic name: zanamivir), Rapivab (generic name: peramivir), and Xofluza (generic name: baloxavir marboxil). Tamiflu may come as a pill or liquid suspension (2021).
This medication may be given to prevent flu in people who have been exposed but do not yet experience symptoms. It must be noted that Tamiflu does not treat the common cold and should not be used as a replacement of annual flu shots. The possible side effects of Tamiflu include vomiting, nausea, and headache. The rare side effects include delirium, hallucinations, sudden confusion, tremors, self-injury, or unusual behavior (Sinha, 2021).
Regarding COVID-19, the so-called “Tamiflu approach” has been described as advantageous as it is more accessible and preventive. People who take this antiviral medication have lower chances of developing serious illness. It may also address emerging SARS-CoV-2 variants with spike protein mutations (Hackethal, 2021).
Difference between Remdesivir and Tamiflu
Definition
Remdesivir (brand name: Veklur) is intravenously administered and was officially approved for the treatment of COVID-19 in October 2020. Initially, remdesivir was created as a possible treatment for hepatitis; it was then later studied as a potential treatment for the Ebola virus. Succeeding research proved that it was effective against Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). In comparison, Tamiflu (generic name: oseltamivir phosphate) is a prescription medicine for the treatment of influenza and may come as a pill or liquid suspension and is used off-label for COVID-19.
Side Effects
The side effects of remdesivir include nausea, pain, bruising, bleeding, or swelling near the place where the solution was injected. Some may also experience serious side effects such as yellow skin or eyes, dark urine, and pain in the upper right of the stomach area (U.S. National Library of Medicine, 2020). On the other hand, the possible side effects of Tamiflu include vomiting, nausea, and headache. The rare side effects include delirium, hallucinations, sudden confusion, tremors, self-injury, or unusual behavior (Sinha, 2021).
COVID-19
Regarding COVID-19, a study showed that remdesivir was superior to placebo in shortening recovery time among hospitalized adult patients. The patients who received the anti-viral drug also had evidence of lower respiratory tract infection (Beigel et al., 2020). As for the so-called “Tamiflu approach” to treating COVID-19, it has been described as advantageous as it is more accessible and preventive. People who take this antiviral medication have lower chances of developing serious illness. It may also address emerging SARS-CoV-2 variants with spike protein mutations (Hackethal, 2021).
Remdesivir vs Tamiflu
Frequently Asked Questions:
Is Remdesivir authorized in Canada for the treatment of severe COVID-19 conditions?
Yes, remdesivir is the first drug that has been authorized by Health Canada for treating COVID-19. It can be used for adults and adolescents who are at least 12 years old with a body weight of at least 40 kilograms (Public Health Agency of Canada, 2020).
What drugs for the coronavirus disease treatment are considered “off-label” use?
The prescription of off-label medications may be subject to national laws and regulations (World Health Organization, 2020). The FDA-Approved drugs used off-label for the treatment of COVID-19 include the following (clinicaltrials.gov, 2020):
- Hydroxychloroquine sulfate
- Lopinavir; ritonavir
- Chloroquine phosphate
- Nitric acid inhaled gas
- Tocilizumab
- Methylprednisolone
- Sarilumab
- Oseltamivir
- Ascorbic Acid
- Ritonavir
- Colchicine
- Ribavarin
- Darunavir; cobicistat
What are some preventative measures for COVID-19?
The World Health Organization specifies ways on preventing the spread of the virus; they include the following:
- Clean your hands often; use soap and water, or an alcohol-based rub
- Observe physical distancing
- Wear masks
- Get vaccinated
- Do not touch your eyes, nose, or mouth
- Stay home if you feel unwell
- Seek medical attention if you have fever, cough and difficulty breathing
Can hand sanitizers kill COVID-19?
According to the Center for Infectious Disease Research Policy:
WHO-recommended alcohol-based sanitizers are effective in killing COVID-19 virus. There are two recommended formulas:
- 80% ethanol, 1.45% glycerol, and 0.125% hydrogen peroxide
- 75% 2-propanol, 1.45% glycerol, and 0.125% hydrogen peroxide
However, the formulations did not meet the European Norm 1500 effectiveness requirements. Hence, the following modified formulations were made by Suchomel and colleagues (Beusokekom, 2020)
- 80% ethanol, 0.725% glycerol, and 0.125% hydrogen peroxide
- 75% 2-propanol, 0.725% glycerol, and 0.125% hydrogen peroxide
Can you recover from COVID-19 on your own?
Yes, most individuals will recover from COVID-19 by themselves, without being hospitalized (Avert, 2021). The symptoms tend to go away after two to three weeks, especially for those with strong immune systems.
Summary
- Remdesivir (brand name: Veklur) is intravenously administered and was officially approved for the treatment of COVID-19 in October 2020.
- Tamiflu (generic name: oseltamivir phosphate) is a prescription medicine for the treatment of influenza and may come as a pill or liquid suspension and is used off-label for COVID-19.
- The side effects of remdesivir include nausea, and pain, bruising, bleeding, or swelling near the place where the solution was injected while those of Tamiflu include vomiting, nausea, and headache.
- Difference Between Hematoma and Melanoma - February 9, 2023
- Difference Between Bruising and Necrosis - February 8, 2023
- Difference Between Brain Hematoma and Brain Hemorrhage - February 8, 2023
Search DifferenceBetween.net :
Leave a Response
References :
[0]Avert. (2021). Corona virus FAQS. https://www.avert.org/coronavirus/faqs
[1]Beigel, J.et al. (2020). Remdesivir for the Treatment of Covid-19- Final report. The New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/nejmoa2007764
[2]Beusekom, V. (2020). Studies: Hand sanitizers kill COVID-19 virus,e-consults appropriate. Center for Infectious Disease Research and Policy. https://www.cidrap.umn.edu/news-perspective/2020/04/studies-hand-sanitizers-kill-covid-19-virus-e-consults-appropriate
[3]Centers for Disease Control and Prevention. (2021). What you should know about flu antiviral drugs. https://www.cdc.gov/flu/treatment/whatyoushould.htm
[4]Hackensack Meridian Health. (2020).What is Remdesivir and how is it used for COVID-19? Health U. https://www.hackensackmeridianhealth.org/HealthU/2020/05/19/what-is-remdesivir-how-is-it-used-for-covid-19/
[5]Hackethal, V. (2021). The Tamiflu approach to treating COVID-19. MedPage Today. https://www.medpagetoday.com/special-reports/exclusives/91702
[6]Public Health Agency of Canada (2020). Remdesivir authorized with conditions for the treatment of patients in Canada with severe COVID-19 symptoms. Government of Canada. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/73621a-eng.php
[7]Sinha, S. (2021). Tamiflu. Drugs.com. https://www.drugs.com/tamiflu.html
[8]U.S. National Library of Medicine. (2019). Remdesivir Injection. Medline Plus. https://medlineplus.gov/druginfo/meds/a620033.html
[9]World Health Organization. (2020).Off-label use of medicines for COVID-19. https://www.who.int/news-room/commentaries/detail/off-label-use-of-medicines-for-covid-19
[10]Image credit: https://commons.wikimedia.org/wiki/File:Tamiflu.jpg
[11]Image credit: https://live.staticflickr.com/65535/50563319461_6318041f7c_b.jpg
